One of the effects of ultrasound is cavitation, or microbubble formation and collapse.
Cavitation produces high pressures and temperatures, and microbubble expansion and then collapse close to cells can lead
to cellular damage or hemorrhage in biological tissues.
If ultrasound and microbubbles could open channels to allow systemically administered drugs into cancer cells, could the technology do more?
https://www.news-medical.net/life-sciences/What-are-Microbubbles.aspx
Microbubbles in cancer
Currently, chemotherapy drugs are injected intravenously and circulate in the patient’s bloodstream. Although they are designed to destroy cancer cells, they also damage healthy tissue, leading to side effects such as nausea and hair loss.
Scientists started to use microbubbles for the targeted release of drugs, which would require significantly smaller doses than when chemotherapy drugs alone are used.
The process involves a microbubble being loaded with a drug and antibodies that target cancer cells. The microbubbles are mixed with water and injected intravenously and tracked by ultrasound until they reach tumor tissue. The frequency of the ultrasound waves is then increased to agitate the bubbles,
which burst
and deliver the drug directly to the tumor cells.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2889676/ Microbubble Compositions, Properties and Biomedical Applications - PMC (nih.gov)
Gene and Drug Delivery
With the exception of contrast enhancement and molecular imaging, the utilization of microbubbles as targeted delivery vehicles is one of the most intensely researched applications of ultrasound contrast agents. Cavitation of bubbles in an ultrasound field can increase the permeability of an endothelial vasculature, allowing small molecules to enter into tissue from the blood stream, a technique known as sonoporation64.
Targeted drug delivery can be accomplished by the incorporation of small molecules into the microbubble shell. The drug can be released upon destruction of the microbubble through ultrasound-mediated cavitation. Ultrasound focusing allows exquisite tissue selectivity. The same rationale for conjugating nucleic acids to microbubbles for gene delivery also applies to molecules for drug delivery applications. Unlike nucleic acids for gene delivery applications, drugs are rarely electrostatically bound to the microbubble surface. Rather, they are incorporated within or just beneath the microbubble shell. Alternatively, they can be loaded into a carrier which can then be linked to the microbubble surface. The articles presented in this review focus on how microbubbles of various shell types are utilized in different drug delivery applications.
http://web.archive.org/web/20141028130550/https://alumni.leeds.ac.uk/bubblemagic
Bring in the first commercial chip-based microfluidic microbubble generating machine in the world, developed right here at Leeds. The HORIZON is portable, practical and speedy – making 109 bubbles in 3 minutes.
It comes with a plastic chip, around the size of a microscope slide, which has tiny channels engraved on it. Different constituents are sent down different channels until they self assemble around a gas bubble. Each microscopic bubble has a consistent size and dosage.
"Our machine opens boundless opportunities," says Professor Evans. "Just like what happened with computer processing, drug making will become miniaturised. Both kinds of chip have very small channels where small amounts are manipulated and controlled."
The same technology could be used, for example, for an early detection system for cancer cells or for food, cosmetics and water-based paint.
https://www.nanowerk.com/spotlight/spotid=43828.php Graphene coated microbubbles as superior photoacoustic imaging contrast agent (nanowerk.com)
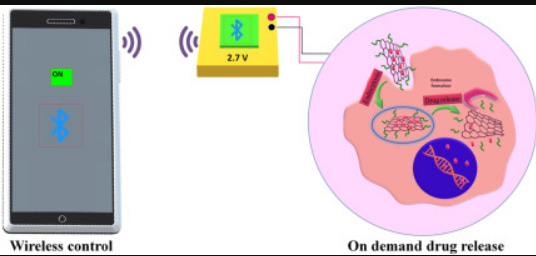
https://www.sciencedirect.com/science/article/abs/pii/S2468519422002166?utm_source=substack&utm_medium=email Remotely controlled electro-responsive on-demand nanotherapy based on amine-modified graphene oxide for synergistic dual drug delivery - ScienceDirect
Many smart materials are used for on-demand drug delivery by utilizing their responses to various stimulations such as temperature, UV light, magnetic, and electrical stimulation. These materials have fewer side-effects and can trigger the drug release by imposing the abovementioned stimulations.
GO is well dispersed in an aqueous solution due to the presence of hydroxyl, carboxyl, and epoxy groups, which significantly enhance the interfacial bonding within the components, and transfer stress efficiently. These advantages make GO an extremely potential nanocomposite material as a drug carrier in the field [11,12] of biomedicine and biotechnology, while being combined with a polymer or inorganic matrix [13,14].
https://youtube.com/watch?v=h9LX4h79aHg&si=cu6BXQps3NKt0iB8
The Destruction Process of Microbubbles in Proximity to Cancer Cells Using Ultrasonic Waves
The videos illustrate the expansion, contraction, and destruction of microbubbles in proximity to cancer cells, and the mechanical impact of this process on the cells.
Advanced Medical Equipment Fields
・ The drug release process in drug delivery systems
・ The generation and disappearance process of microbubbles, which are utilized for sterilization and ultrasound diagnosis
In medical treatment and biotechnology fields, research is advancing using the dynamics of so-called microbubbles, microscopic bubbles on the order of 1 to 100 microns.
When microbubbles in a fluid are exposed to ultrasonic waves, they expand, contract, and then disappear, a process that generates a localized, high-speed flow referred to as a microjet. Research is being performed regarding the use of this phenomenon to open pores in cells so as to introduce genes and pharmaceutical agents directly into cells.
Microbubbles are extremely minute, so the process of expansion, contraction, and destruction occurs at very high speeds. Accordingly, a high-sensitivity, high-speed camera is required to analyze this behavior.
In addition, high-speed cameras are used to observe the behavior of ultrasonic waves from ultrasonic generators.
The Destruction Process of Microbubbles in Proximity to Cancer Cells Using Ultrasonic Waves
Research is advancing into a drug delivery system in which microcapsules containing pharmaceutical agents and microbubbles are introduced in proximity to cancer cells. Exposure to ultrasonic waves is used to rupture the capsules, and the pharmaceutical agents are then guided into the cancer cells. The images illustrate the expansion, contraction, and destruction of microbubbles in proximity to cancer cells, and the mechanical impact of this process on the cells.
(Provided by Division of Bioengineering and Bioinformatics at Hokkaido University)
Recording speed: 10 million frames/second
Width of field of view: Approx. 130 μm
High-Speed Contraction of Microbubbles
The images illustrate the contraction and disappearance of microbubbles resulting from an electrical discharge at the tip of a microscopic tube. Research is being conducted into micro-scalpels and other applications using the high-speed flow generated when microbubbles disappear.
(Provided by the Yamanishi Laboratory at the Shibaura Institute of Technology)
Recording speed: 1 million frames/second
Width of field of view: Approx. 0.2 mm
https://link.springer.com/article/10.1007/s40846-018-0391-0 Ultrasound Cavitation/Microbubble Detection and Medical Applications | SpringerLink
The presence of microbubbles in the human body can be induced either through cavitation or exogenous introduction of bubbles.
One of the effects of ultrasound is cavitation, or microbubble formation and collapse. Cavitation produces high pressures and temperatures, and microbubble expansion and then collapse close to cells can lead
to cellular damage or hemorrhage in biological tissues.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9340509/ Prediction of vascular injury by cavitation microbubbles in a focused ultrasound field - PMC (nih.gov)
Abstract
Many studies have shown that microbubble cavitation is one mechanism for vascular injury under ultrasonic excitation. Previous work has attributed vascular damage to vessel expansions and invaginations due to the expansion and contraction of microbubbles. However, the mechanisms of vascular damage are not fully understood. In this paper, we investigate, theoretically and experimentally, the vessel injury due to stress induced by ultrasound-induced cavitation (UIC). A bubble-fluid-vessel coupling model is constructed to investigate the interactions of the coupling system. The dynamics process of vessel damage due to UIC is theoretically simulated with a finite element method, and a focused ultrasound (FU) setup is carried out and used to assess the vessel damage. The results show that shear stress contributes to vessel injury by cell detachment while normal stress mainly causes distention injury. Similar changes in cell detachment in a vessel over time can be observed with the experimental setup. The severity of vascular injury is correlated to acoustic parameters, bubble-wall distance, and microbubble sizes, and the duration of insonation.
https://pubs.aip.org/asa/jasa/article-abstract/124/4/2374/980885/Ultrasonic-excitation-of-a-bubble-inside-a?redirectedFrom=fulltext Ultrasonic excitation of a bubble inside a deformable tube: Implications for ultrasonically induced hemorrhage | The Journal of the Acoustical Society of America | AIP Publishing
Various independent investigations indicate that the presence of microbubbles within blood vessels may increase the likelihood of ultrasound-induced hemorrhage.
https://tecknexus.com/5gusecase/remote-controlled-ultrasound-scan-over-a-public-5g-network/
IS HEMORRHAGIC FEVER SUPPOSED TO BE THE RESULT OF ULTRASOUND-INDUCED CAVITATION OF MICROBUBBLES?
IS THIS WHAT THE ALLEGED SECOND PANDEMIC IS ALL ABOUT?
Notice of Declaration Under the Public Readiness and Emergency Preparedness Act for Countermeasures Against Marburgvirus and/or Marburg Disease
A Notice by the Health and Human Services Department on 12/09/2020
Declaration
Declaration for Public Readiness and Emergency Preparedness Act Coverage for Countermeasures Against Marburgvirus and/or Marburg Disease
I. Determination of Public Health Emergency
42 U.S.C. 247d-6d(b)(1)
I have determined that Marburg disease and marburgviruses are a credible risk such that Marburg disease or marburgviruses may in the future constitute a public health emergency. This Declaration must be construed in accordance with the Advisory Opinions of the Office of the General Counsel (Advisory Opinions). I incorporate those Advisory Opinions as part of this Declaration.[] This Declaration is a “requirement” under the PREP Act.
II. Factors Considered
42 U.S.C. 247d-6d(b)(6)
I have considered the desirability of encouraging the design, development, clinical testing, or investigation, manufacture, labeling, distribution, formulation, packaging, marketing, promotion, sale, purchase, donation, dispensing, prescribing, administration, licensing, and use of the Covered Countermeasures.
III. Recommended Activities
42 U.S.C. 247d-6d(b)(1)
I recommend, under the conditions stated in this Declaration, the manufacture, testing, development, distribution, administration, and use of the Covered Countermeasures.
IV. Liability Immunity
42 U.S.C. 247d-6d(a), 247d-6d(b)(1)
Liability immunity as prescribed in the PREP Act and conditions stated in this Declaration is in effect for the Recommended Activities described in Section III.
V. Covered Persons
42 U.S.C. 247d-6d(i)(2), (3), (4), (6), (8)(A) and (B)
Covered Persons who are afforded liability immunity under this Declaration are “manufacturers,” “distributors,” “program planners,” “qualified persons,” and their officials, agents, and employees, as those terms are defined in the PREP Act, and the United States. In addition, I have determined that the following additional persons are qualified persons: (a) Any person authorized in accordance with the public health and medical emergency response of the Authority Having Jurisdiction, as described in Section VII below, to prescribe, administer, deliver, distribute or dispense the Covered Countermeasures, and their officials, agents, employees, contractors and volunteers, following a Declaration of an emergency; (b) any person authorized to prescribe, administer, or dispense the Covered Countermeasures or who is otherwise authorized to perform an activity under an Emergency Use Authorization in accordance with Section 564 of the FD&C Act; and (c) any person authorized to prescribe, administer, or dispense Covered Countermeasures in accordance with Section 564A of the FD&C Act.
VI. Covered Countermeasures
42 U.S.C. 247d-6b(c)(1)(B), 42 U.S.C. 247d-6d(i)(1) and (7)
Covered Countermeasures are any antiviral, any other drug, any biologic, any diagnostic, any other device, or any vaccine, used to treat, diagnose, cure, prevent, or mitigate Marburg disease, or the transmission of marburgviruses or a virus mutating therefrom, or any device used in the administration of any such product, and all components and constituent materials of any such product, or countermeasures for adverse effects of these countermeasures, and countermeasures that otherwise limit the harm caused by the health threat.
Covered Countermeasures must be “qualified pandemic or epidemic products,” or “security countermeasures,” or drugs, biological products, respiratory protective devices or devices authorized for investigational or emergency use, as those terms are defined in the PREP Act, the FD&C Act, and the Public Health Service Act.
VII. Limitations on Distribution
42 U.S.C. 247d-6d(a)(5) and (b)(2)(E)
I have determined that liability immunity is afforded to Covered Persons only for Recommended Activities involving Covered Countermeasures that are related to:
(a) Present or future federal contracts, cooperative agreements, grants, other transactions, interagency agreements, memoranda of understanding, or other federal agreements; or
(b) Activities authorized in accordance with the public health and medical response of the Authority Having Jurisdiction to prescribe, administer, deliver, distribute or dispense the Covered Countermeasures following a Declaration of an emergency.
As used in this Declaration, the terms Authority Having Jurisdiction and Declaration of Emergency have the following meanings:
i. The Authority Having Jurisdiction means the public agency or its delegate that has legal responsibility and authority for responding to an incident, based on political or geographical (e.g., city, county, tribal, state, or federal boundary lines) or functional (e.g., law enforcement, public health) range or sphere of authority.
ii. A Declaration of Emergency means any Declaration by any authorized local, regional, state, or federal official of an emergency specific to events that indicate an immediate need to administer and use the Covered Countermeasures, with the exception of a federal Declaration in support of an Emergency Use Authorization under Section 564 of the FD&C Act unless such Declaration specifies otherwise;
I have also determined that, for governmental program planners only, liability immunity is afforded only to the extent such program planners obtain Covered Countermeasures through voluntary means, such as (1) donation; (2) commercial sale; (3) deployment of Covered Countermeasures from federal stockpiles; or (4) deployment of donated, purchased, or otherwise voluntarily obtained Covered Countermeasures from state, local, or private stockpiles.
VIII. Category of Disease, Health Condition, or Threat
42 U.S.C. 247d-6d(b)(2)(A)
The category of disease, health condition, or threat for which I recommend the administration or use of the Covered Countermeasures is Marburg disease caused by marburgviruses or virus mutating therefrom.
IX. Administration of Covered Countermeasures
42 U.S.C. 247d-6d(a)(2)(B)
Administration of the Covered Countermeasure means physical provision of the countermeasures to recipients, or activities and decisions directly relating to public and private delivery, distribution and dispensing of the countermeasures to recipients, management and operation of countermeasure programs, or management and operation of locations for purpose of distributing and dispensing countermeasures.
X. Population
42 U.S.C. 247d-6d(a)(4), 247d-6d(b)(2)(C)
The populations of individuals include any individual who uses or is administered the Covered Countermeasures in accordance with this Declaration.
Liability immunity is afforded to manufacturers and distributors without regard to whether the countermeasure is used by or administered to this population; liability immunity is afforded to program planners and qualified persons when the countermeasure is used by or administered to this population, or the program planner or qualified person reasonably could have believed the recipient was in this population.
XI. Geographic Area
42 U.S.C. 247d-6d(a)(4), 247d-6d(b)(2)(D)
Liability immunity is afforded for the administration or use of a Covered Countermeasure without geographic limitation.
Liability immunity is afforded to manufacturers and distributors without regard to whether the countermeasure is used by or administered in any designated geographic area; liability immunity is afforded to program planners and qualified persons when the countermeasure is used by or administered in any designated geographic area, or the program planner or qualified person reasonably could have believed the recipient was in that geographic area.
XII. Effective Time Period
42 U.S.C. 247d-6d(b)(2)(B)
Liability immunity for Covered Countermeasures through means of distribution, as identified in Section VII(a) of this Declaration, other than in accordance with the public health and medical response of the Authority Having Jurisdiction and extends through August 1, 2025.
Liability immunity for Covered Countermeasures administered and used in accordance with the public health and medical response of the Authority Having Jurisdiction begins with a Declaration and lasts through (1) the final day the emergency Declaration is in effect, or (2) August 1, 2025, whichever occurs first.
XIII. Additional Time Period of Coverage
42 U.S.C. 247d-6d(b)(3)(B) and (C)
I have determined that an additional 12 months of liability protection is reasonable to allow for the manufacturer(s) to arrange for disposition of the Covered Countermeasure, including return of the Covered Countermeasures to the manufacturer, and for Covered Persons to take such other actions as are appropriate to limit the administration or use of the Covered Countermeasures.
Covered Countermeasures obtained for the SNS during the effective period of this Declaration are covered through the date of administration or use pursuant to a distribution or release from the SNS.















TMI.
Between the medicine man, and the 5G experts, we are going down the wrong road to the extermination of life on earth !
We need to stop this madness now, and stop trying to improve on gods design, and just embrace our earth and respect it !
They just confirmed all the so-called crazy vaccine conspiracies in a single paper 👏