Funded by the Bill and Melinda Gates Foundation in 2017:
https://www.gatesfoundation.org/about/committed-grants/2017/09/opp1183206
Committed grants: Integrated Nano-Technologies (INT)
Purpose: To assess novel, low cost nucleic acid detection technologies for use in low resource settings
GRANTEE WEBSITE:
http://web.archive.org/web/20180318184930/http://integratednano.com/ Integrated Nano-Technologies (archive.org)
YES, THAT’S RIGHT:
AVAILABLE DIAGNOSTIC TESTS
• Bovine Viral Diarrhea virus (BVD)
• West Nile virus
• Anthrax
• Alpha viruses
• Flavi viruses
About Integrated Nano-Technologies (INT)
DEVELOPING & COMMERCIALIZING RAPID DIAGNOSTICS
INT is committed to providing field personnel with fast, accurate test results that can improve biological identification. INT was founded in 2000 by an interdisciplinary team of scientists and engineers to develop creative solutions for diagnostics sample processing. Through government funding and private investments, INT has developed a working electronic DNA sensor, a universal sample preparation system and a novel rotary valve disposable cartridge for automated processing of complex biological analysis.
This working system is in validation studies with a number of commercial and government customers.
http://web.archive.org/web/20170627081243/http://www.integratednano.com/about/leadership.aspx
Integrated Nano-Technologies Leadership (INT)
INT is led by Chairman and CEO D. Michael Connolly, supported by a board of legal, financial, scientific, military and medical experts. Their involvement ensures that INT develops the Palladium™ System to meet current and emerging market needs, on a sound corporate footing.
Management Team
D. MICHAEL CONNOLLY, JR., PHD, JD
CEO
Dr. Connolly is the Chairman of the Board. He is also President and Chief Executive Officer of the Company. He has a PhD in molecular biology from Northwestern University and completed a post-doctoral fellowship in the Department of Microbiology at the University of Illinois Medical Center. After completing his JD at Cornell University, he practiced as a patent attorney at Nixon Peabody, LLP in Rochester. While at Nixon Peabody, he played a significant role in developing the licensing strategy for a major genetic sequencing enterprise, as well as patenting and licensing technologies for several other companies and universities.
ROBERT LATELLA
COO
Robert N. Latella joined the company as Chief Operating Officer in June 2009. Mr. Latella also serves as Of Counsel to the law firm of Hiscock & Barclay, LLP. He has extensive experience in multiple disciplines including law, finance and management, having previously served as the Chief Operating Officer of the Genesee Corporation (a diversified public company), where he had chief executive responsibility for a number of business units, and as Chief Financial Officer of The Case Hoyt Corporation. Mr. Latella commenced his legal career at the law firm of Harter, Secrest & Emery and served in several roles including service as the firm’s Managing Partner. He has broad experience with start-up entities and the implementation of capital formation strategies. He currently serves on several boards of directors including Financial Institutions, Inc.(Nasdaq: FISI) and the University of Rochester Medical Center where he is Chair Elect. He is a graduate of Fordham College and earned law degrees from Vanderbilt University (LL.B) and New York University School of Law (LL.M. in Corporate Law).
JANE W. BRYAN
CONTROLLER
Ms. Bryan joined the Company as Controller in October 2007. Her financial background is varied. She held several positions at the Rochester Institute of Technology in the areas of Sponsored Programs Accounting for grants and contracts, Business Process, Financial Analysis and Internal Audit. While at RIT she also worked on the design and installation of Oracle applications and was the in-house functional manager for two of the applications. At CNA Financial she was Manager of Financial Policy. Jane also has several years of experience as a CPA with Deloitte & Touche. She is a cum laude graduate of Boston University.
MARK J. SMITH
DIRECTOR OF MANUFACTURING OPERATIONS
Mr. Smith is the Director of Manufacturing Operations at the company. Prior to joining the company, he was the Director of Worldwide Supply Chain and Business Operations for the Consumer Inkjet Division of the Eastman Kodak Company. Mr. Smith held a variety of leadership positions with Kodak over the course of a 35 year career, including executive roles in manufacturing, engineering, and development organizations. He received a B.S. in Chemical Engineering from Michigan State University and a certificate in Manufacturing Management from the Darden School at the University of Virginia.
Senior Research Scientists
DR. DAVID B. BAILEY
Dr. Bailey joined the Company as a Senior Research Scientist in 2007 after 32 years at the Eastman Kodak Research Laboratories, where he developed materials for imaging, printing and microelectronic technologies. He is currently applying his synthetic polymer chemistry expertise to metal development chemistry to improve the shelf life of the components and the overall sensitivity and specificity of the system. He received his PhD in Polymer Science and Engineering from the University of Massachusetts at Amherst.
DR. RICHARD S. MURANTE
Dr. Murante is a Senior Research Scientist with the company. He has played a pivotal role in the development of the technology. His area of specialty is biochemistry and molecular biology. He received his Ph.D. in Biochemistry from the University of Rochester. He later went on to be a Research Assistant Professor at the University of Rochester before coming to the Company in 2000. Dr. Murante has multiple publications in the field of molecular biology. He has experience in the genetic computing field and has served as the Principal Investigator on a number of significant federal awards.
NATE WESCOTT
Nate Wescott, Design and Process Engineer, joined INT in 2001 to lead the development of chip design and processes. His materials research has led to increased system sensitivity through Palladium’s proprietary microfluidic card design. He received his M.S. and B.S. in Microelectronic Engineering from Rochester Institute of Technology, and his work has been published by SPIE, IEEE, and others.
Board of Directors
DAVID R. FRANZ, DVM, PHD
Dr. Franz serves as Vice President and Chief Biological Scientist of the Midwest Research Institute. He is also senior advisor to the Office of the Assistant to the Secretary of Defense for Nuclear, Chemical and Biological Defense programs. Dr. Franz served in the U.S. Army Medical Research and Materiel Command for 23 of 27 years on active duty and retired as Colonel. He served as commander of the U.S. Army Medical Research Institute of Infectious Diseases and as deputy commander of the Medical Research and Materiel Command. Prior to joining the Command, he served as group veterinarian for the 10th Special Forces Group (Airborne). He was the chief inspector on three United Nations Special Commission biological warfare inspection missions to Iraq and served as technical advisor on long-term monitoring. He also served as a member of the first two U.S.-U.K. teams that visited Russia in support of the Trilateral Joint Statement on Biological Weapons and as a member of the Trilateral Experts' Committee for biological weapons negotiations. Dr. Franz earned a DVM from Kansas State University and a Ph.D. in physiology from Baylor College of Medicine.
DENNIS M. CONNOLLY, SR., JD
Mr. Connolly is an attorney in Omaha, Nebraska. He brings experience in both law and business to the Board of Directors. His private practice of more than 30 years has focused on business-related law. He received his BS and JD from Creighton University and served on the Board of Directors for the Nebraska National Bank, was a regent for Conception Seminary College, and was a regional director for USA-Canada Serra Council and trustee of Serra International Foundation. Mr. Connolly has served as counsel for Mount Michael Benedictine Abbey and High School and was active on the boards of several private high schools and clubs in the Omaha area.
J. MICHAEL HOLLOWAY
Mr. Holloway retired from the financial services industry in 2004 after 31 years with two institutions including 13 years at Citicorp and 18 years at Rochester Community Savings Bank/Charter One Financial/Citizens Financial. His career was equally split between that of a business banker and in executive management as a senior business line manager. In his role as a senior executive manage,r Mr. Holloway was the CEO of a multi-state commercial real estate investment and development business and a national automobile lending business. He has held board and/or leadership positions with the Consumer Bankers of America, the Rochester Museum and Science Center, the Greater Rochester Chapter of the American Red Cross, St. John of Rochester Parish/The Roman Catholic Diocese of Rochester, and Cornell University. Mr. Holloway is a graduate of Cornell University.
JOHN M. NOONAN
Mr. Noonan is a Research Chemist at the company. Prior to joining the company, he worked as a Senior Research Associate at Eastman Kodak Research Laboratories for over 35 years. He also worked for Corning Glass Works Research Labs as a Research Chemist. He received his M.S. in Organic Chemistry from the University of Delaware and his B.S. in Chemistry from St. Bonaventure University.
GENERAL DENNIS J. REIMER (U.S. ARMY, RET.)
General Dennis J. Reimer (U.S. Army, Ret.) served over 37 years in the U. S. Army. In 1995, General Reimer became the 33rd Chief of Staff, U.S. Army and a member of the Joint Chiefs of Staff, the primary military advisory group to the President of the United States. Prior to that, he was the Commanding General of the United States Army, Forces Command, Fort McPherson, Georgia. During his military career he served in a variety of other joint and combined assignments, as well as two combat tours in Vietnam. He also served in Korea as the Chief of Staff, Combined Field Army and Assistant Chief of Staff for Operations and Training, Republic of Korea/United States Combined Forces Command. After his retirement from the army in 1999 General Reimer became the first Director of the National Memorial Institute for the Prevention of Terrorism in Oklahoma City, an organization dedicated to preventing terrorism in the United States. In 2005 he returned to Washington D.C. to take a position with DFI, a national security consulting firm. DFI was acquired by a British firm, Detica, and General Reimer remained with the new organization for about a year. He serves on the Mutual of America, DRS Technologies and Virtual Agility Boards. He is also the President of Army Emergency Relief and a trustee for the Association of the United States Army.
One could say - quite a company....
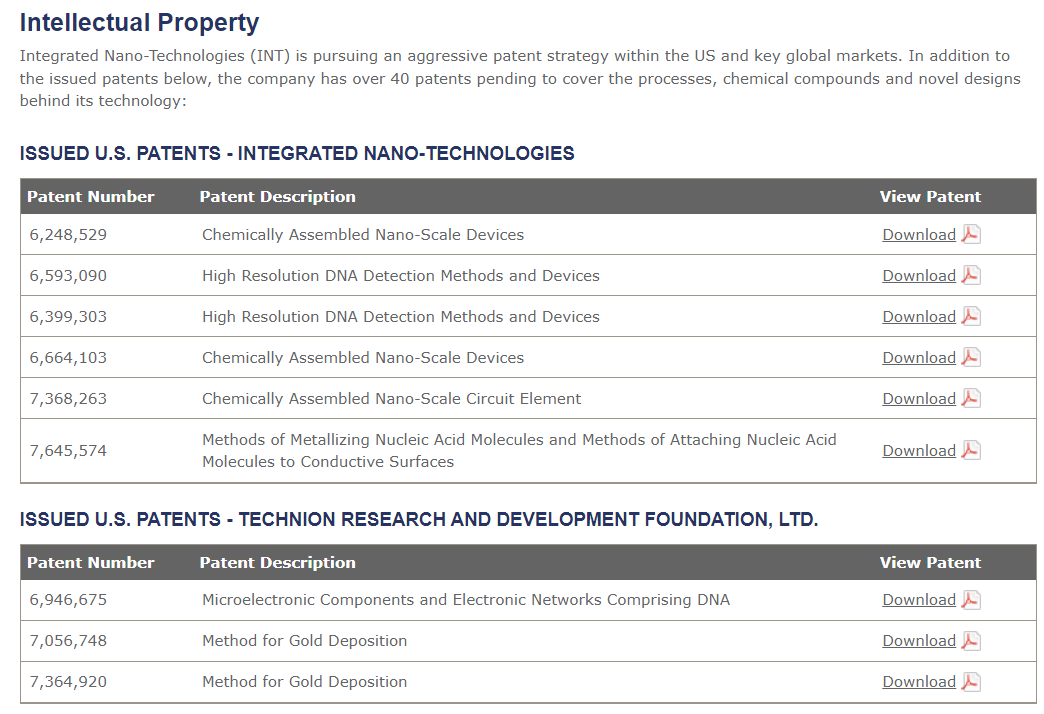
http://web.archive.org/web/20170628025251/http://integratednano.com/about/intellectual-property.aspx
http://web.archive.org/web/20161202064035/http://integratednano.com/pdf/US-07368263_1.pdf
http://web.archive.org/web/20161202064043/http://integratednano.com/pdf/US-6593090.pdf
http://web.archive.org/web/20161201134950/http://integratednano.com/pdf/US-6399303.pdf
http://web.archive.org/web/20161201205113/http://integratednano.com/pdf/US-6664103-.pdf
http://web.archive.org/web/20160809214720/http://integratednano.com/pdf/US7645574.pdf
http://web.archive.org/web/20161201182830/http://integratednano.com/pdf/US-6946675-.pdf
http://web.archive.org/web/20161201114703/http://integratednano.com/pdf/US-07056748_1.pdf
http://web.archive.org/web/20170627194413/http://integratednano.com/applications/diagnostics.aspx
And so on…
Later this website changed into:
http://web.archive.org/web/20210621014626/https://www.intpalladium.com/ Integrated Nano-Technologies (archive.org)
The Palladium™ COVID Test System is a PCR-based test based on novel, proprietary technology. It integrates sample prep, PCR amplification and detection in a closed system for unparalleled ease-of-use. There is absolutely no sample prep required - it’s as easy as sample in and results out in approximately 2 hours .*
*Not FDA cleared. Not yet available for sale.
And this is this website now:
https://www.intpalladium.com/ Integrated Nano Technologies (intpalladium.com)


















thank you
"PCR amplification and detection in a closed system for unparalleled ease-of-use...no sample prep required"
This means that the result is just a pre-written print out. A 70% positive rate sounds about right.
Same as metagenomics: nothing in---pre-written answer out.