Ingestible sensors
Before I move on to the Microchipped and marked - PART 3, I would like to write a few words about microchip technology (ingestible electronics) in pills:
Did you take your pill? Ingestible sensors can tell
Andrew Thompson grabbed a water bottle and swallowed a small white pill.
It’s a mundane act that humans have done for centuries. But what occurred a few minutes later was very 21st century: A notification popped up on his iPhone indicating that he had, in fact, taken the pill.
“We’re the only company in the world that can do this,” said Thompson, CEO of Proteus Digital Health of Redwood City, as the pill — which has a tiny sensor in it — funneled down to his stomach.
Some experts say ingestibles — also dubbed “smart pills” — can help solve one of the biggest problems plaguing the health care industry:
patients simply not taking their medication as prescribed.
The Proteus sensor can indicate if and when a pill is taken. It was approved by the Food and Drug Administration in 2012 — the first such device to receive the agency’s OK. It is sold commercially to nine health systems, three of which are in California. In 2014, federal regulators cleared an ingestible camera that can screen for colon cancer.
Once the pill dissolved in Thompson’s stomach,
a pinhead-size sensor made of copper, magnesium and silicon — ingredients already in our daily diets
— was left behind. The sensor, activated by fluids in the body, sent a signal to a patch on the side of his stomach that also measures heart rate, body position and time of medication detection. His iPhone, linked to the patch, then registered the information.
Kevin Rodondi, an associate professor at UCSF’s Department of Clinical Pharmacy, said
data on whether a pill has been taken could be pivotal for the American health care industry,
which loses billions of dollars a year
from people not taking their medication.
Consumer acceptance will be vital for ingestibles, Rodondi said.
“We need to reinvent health care, and these technologies are going to bridge the ways we do that,” he said.
Perhaps "a sensor the size of a pin head made of copper, magnesium and silicon - ingredients that are already in our daily diet", but we don't chew copper wires, magnesium alloys used in the automotive industry, silicon breasts implants or even silicon contact lenses.
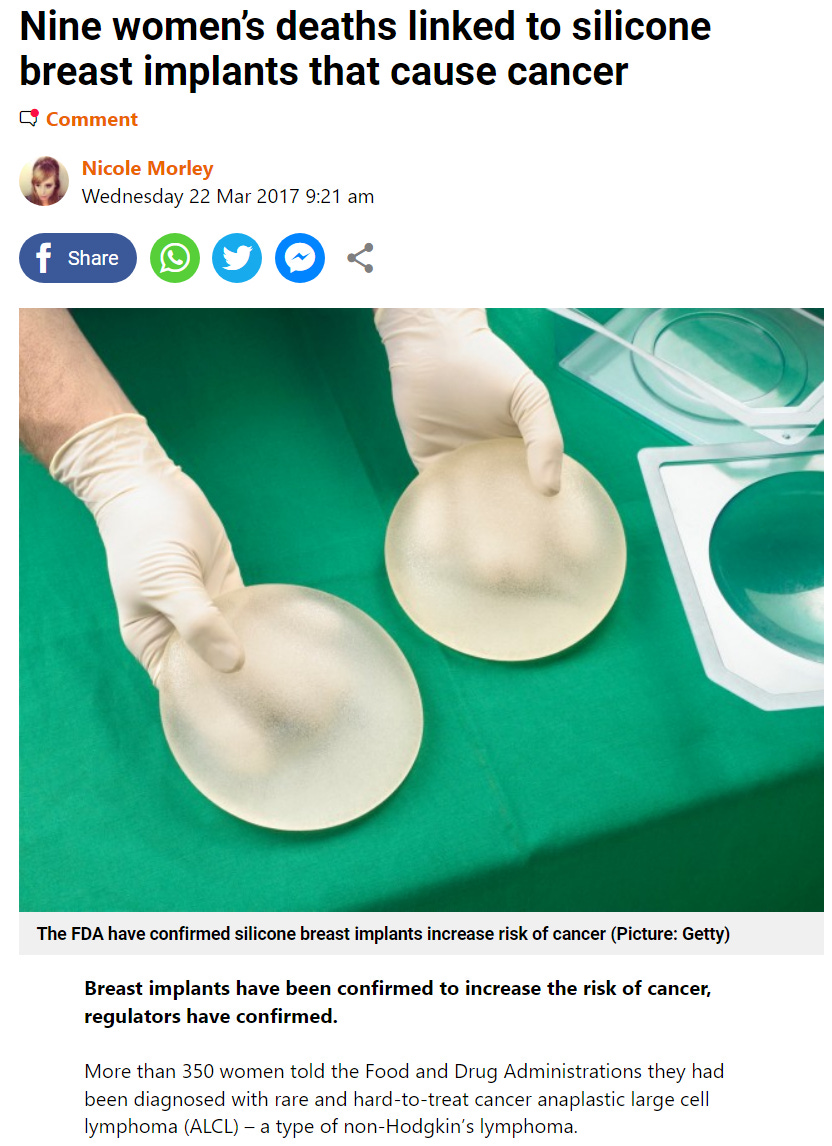
‘Most cases of breast implant-associated ALCL are treated by removal of the implant and the capsule surrounding the implant and some cases have been treated by chemotherapy and radiation.
The statement added: ‘As of February 1, 2017, the FDA has received a total of 359 medical device reports of breast-implant-associated ALCL, including nine deaths,’ it said.
But they were made of silicon, one of the "- ingredients that are already in our daily diet". That's their twisted rhetoric....
Here is also an interesting research article to better understand these technologies:
Digital compliance measurement using an ingestible radio-frequency identification chip. Ingestion is registered by a wearable patch, which gives healthcare providers the possibility to adapt the therapy based on an unbiased data set.
Semiconducting and thermal conductivity sensing elements can be used, which undergo consecutive heating cycles to achieve sensitivity and specificity towards hydrogen, methane and carbon dioxide.
Nanoporous materials, such as metal organic frameworks and carbon nanotubes,
can also be applied gas-sensing applications. For example, these materials can be placed in arrays to monitor gas profiles within the GI (gastrointestinal) tract, including gases originating from volatile organic compounds.
Miniaturization and cost reduction of electronic devices have opened the way for electronic systems in oral drug delivery; for example, preclinical concepts for improved ultrasound-mediated drug permeation and clinical concepts for electronically controlled delivery of active pharmaceutical ingredients are being explored. Such devices with wireless telemetry functionalities can be used to shuttle drugs to preferred absorption sites along the GI tract. They also provide a tool for personalized and integrated delivery concepts; for example, drug delivery systems that reside in the body can automatically release drugs based on a personalized treatment plan.
Chip on a pill.
IEM-enhanced pill, their stomach acids activate the microchip, which then sends data such as heart rate, temperature, and body movements to a dermal patch via Bluetooth connectivity. This patch can then export the data to an EMR, so that it can be accessed by the patient’s doctors. Novartis claims that because their device will not alter the effects of the drugs it is paired with, they could bring the IEM to market in as little as two years.
LEGAL ISSUES
For example, since the use of these sensors is at least initially unfamiliar to patients, it is especially vital to respect the patients’ autonomy by ensuring full and voluntary informed consent. The use of a smartphone app as part of the system also involves click-through user agreements that patients frequently do not read, and if they read them often have problems understanding them. An additional challenge is that these user agreements will be frequently updated.
The legal classification of IESs, and thus the relevant pathway for bringing IESs to market in the United States and Europe, needs to be assessed on a case-by-case basis. There are two types of IESs: those that are co-ingested with medicine and those that are taken as an embedded part of a drug.
Wearable sensors, including IESs used independent of medication, are classified as medical devices in the United States and Europe. For example, the wearable sensor, including the IES, from Proteus Digital Health has been CE-marked (i.e., a necessary condition to place a medical device on the European market) as a Class IIa medical device in Europe since 2010. The United States Food and Drug Administration (FDA) classified the device through the De Novo classification process into Class II and permitted marketing in 2012.
In contrast, a drug product that is physically embedded with an IES such as Abilify MyCite is a drug-device combination product in the United States. Marketing application for such product usually depends on its primary mode of action. Abilify MyCite, for instance, has a drug primary mode of action. Thus, a New Drug Application approval was needed to market Abilify MyCite, which Otsuka Pharmaceutical received by FDA in 2017. In Europe, however, these drug-device “combination products” are classified as medicinal products and their marketing within a Member State (e.g., Germany) typically requires authorization of that Member State’s competent authorities.
In our paper, we also explore new legal developments in the United States and Europe, such as FDA’s recently proposed framework for regulating prescription drug-use-related software, the new EU Medical Device Regulation as well as the new EU General Data Protection Regulation and its impact on United States companies.
First, IESs raise a variety of patient issues—especially the ethical issues of autonomy and informed consent.
Ownership of the data collected by IES products raises a multitude of issues, including the question of the doctor–patient privilege and the related issue of medical confidentiality. The availability of this data in the hands of third parties might have implications on life insurance premiums, employment opportunities, and even personal relationships, depending on the national law of the country where the patient resides. IES makers must be frank about the future use of the collected data and the terms surrounding it. For example, in what way will identified and de-identified data be stored and aggregated? With whom will it be shared? Can patients request that their data be destroyed and do such withdrawal rights apply to data that has already been analysed in aggregate form? To what extent do such rights of withdrawal conflict with potential requirements of post-market surveillance that may be imposed by FDA and other regulators? Finally, obtaining informed consent can be particularly challenging in cases of vulnerable patient groups such as prisoners, or individuals out on probation. When IESs will be used in such contexts, particular care must be taken to make sure the consent is not only informed but also voluntary.
A final set of patient-centred ethical issues concern patient expectations. An IES may enable but not mandate a member of the patient’s care team to access information such as ingestion to determine if the patient has or has not been taking their medication. Patients using an IES may have a different expectation as to whether or how often they are being ‘checked up on’.
Second, IESs also raise provider-centred ethical issues. On the one hand, the hope is that IESs will improve the clinician–patient relationship by enabling the clinician to better understand what is going on (biologically and/or socially) with the patient, and thus facilitate a more open dialogue between both parties. However, there will also
be patients who may feel ‘surveilled’—in the sense of unwanted observation—by their health-care providers through such systems.
When a patient is the one who requests an IES as opposed to the non-IES formulation of the same therapy the voluntariness of the decision is at its zenith. In other cases, the pressure may be subtle or gross. Consider a patient who uses the IES formulation to please his or her family or a patient whose insurer will only cover the IES formulation. These are not easy waters to navigate, but effective use of IES products is built on a trusting doctor–patient relationship, where open dialogue is fostered and not chilled.
While IES systems are designed to primarily gather data on the patient, few physicians will realize at first how much information about the physician or other members of the care team (for example, tracking when a physician logs on) is also collected. To what extent do members of the care team have to consent to the collection and use of their data?
Wearable sensors including IESs by themselves are medical devices in the US and Europe. In the US, a medical device is defined in section 201(h) of the Federal Food, Drug, and Cosmetic Act and ranges from a simple tongue depressor to a complex robotically assisted surgical device to an invitro diagnostic product such as a test kit or reagent. In particular, a medical device—in contrast to a typical drug—“does not achieve its primary intended purposes through chemical action … and … is not dependent upon being metabolized for the achievement of its primary intended purposes”.
FDA regulates medical devices intended for human use in the US and divides them into three classes: Class I (that is, low-risk devices such as patient scales), Class II (that is, moderate-risk devices such as sickle-cell test kits), and Class III (that is, high-risk devices such as replacement heart valves). New devices are automatically placed in Class III. However, the ‘De Novo’ classification process offers sponsors the opportunity to request a risk-based evaluation by FDA for classification of their new devices into Class I or II.
In 2012, FDA classified Proteus’s wearable sensor, including the IES, through the De Novo classification process into Class II under the generic name “Ingestible Event Marker”.
FDA also concluded that devices “substantially equivalent” to the Proteus device are classified as Class II under this generic name. FDA clarified that this device type is not exempt from the premarket notification requirements of the Federal Food, Drug, and Cosmetic Act. Thus, sponsors who intend to market this device type need to submit a Premarket Notification 510(k) before marketing the device and receive ‘clearance’ to market from the agency.
In contrast to Class III devices that require pre-market approval to provide reasonable assurance of their safety and effectiveness, Class II devices are ‘only’ subject to general controls and special controls. General controls, for example, include requirements for establishment registration and medical device listing. Special controls for Ingestible Event Markers such as Proteus’s wearable sensor, including the IES, for example, consist of the following four measures: biocompatibility and toxicology testing; non-clinical, animal, and clinical testing; electromagnetic compatibility, wireless, and electrical safety testing; and special labelling such as the maximum number of daily device ingestions. In addition, Proteus’s device and substantially equivalent devices are prescription devices.
The next generation of digital health products should have an easier entry to the US market, both because the relevant pathways have been used and because FDA has announced a new Digital Health Innovation Action Plan that strives to ensure “timely access to high-quality, safe and effective digital health products”.
In the EU, the Medical Device Directive 93/42/EEC (MDD) applies to medical devices. A directive, in comparison to a regulation, needs to be transposed into national law. On 25 May 2017, the new EU Medical Device Regulation 2017/745 (MDR) entered into force. However, with few exceptions, the MDR will only apply in each member state from 26 May 2020 and repeal the MDD and the Active Implantable Medical Devices Directive 90/385/EEC (AIMD). In addition, at the same time as the MDR, the EU In Vitro Diagnostic Medical Devices Regulation 2017/7 (IVDR) came into force. The IVDR will generally apply two years later than the MDR (that is, from 26 May 2022) and repeal, inter alia, the In Vitro Diagnostic Medical Devices Directive 98/79/EC (IVDD).
Medical devices may only be placed on the market in Europe if they fulfil all CE-marking requirements set out in the relevant directives. A medical device is defined in article 1(2)(a) of the MDD, IVDD, and AIMD, and similar to US law “does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means.” Thus, in contrast to a medicinal product, as defined in article 1(2) of the Medicines Directive 2001/83/EC, a medical device typically acts by physical means. For example, smartphone apps may be classified as medical devices under certain conditions, such as where the software is “intended by the manufacturer to be used for human beings for the purpose of diagnosis, prevention, monitoring, treatment or alleviation of disease ... [or] … an injury or handicap”. Similar to US law, medical device according to the MDD are classified into product classes. In total, there are four classes of medical devices (instead of three classes in US law), taking into account their potential risks: Class I (low risk), Class IIa (medium risk), Class IIb (higher risk), and Class III (highest risk). For example, Proteus’s wearable sensor, including the IES, has been CE-marked as a Class IIa device in Europe since 2010. In addition, medical apps will mostly be classified into Class I, but Class IIa or Class IIb are also possible. In contrast to the US, there is no clear definition of a combination product in the EU. However, article 1(3) of the MDD states that the Medicines Directive shall generally apply in the case of a product that “is placed on the market in such a way that the device and the medicinal product form a single integral product which is intended exclusively for use in the given combination and which is not reusable.” Such a ‘combination product’ is thus classified as a medicinal product and its marketing within an EU member state usually requires the authorization of the competent authorities of that member state.
However, in some cases (such as in the case where the medicinal product component is an advanced therapy medicinal product), the marketing authorization is granted by the European Commission through the so-called centralized procedure and is valid throughout Europe.
‘Data concerning health’ is “personal data related to the physical or mental health of a natural person, including the provision of health care services, which reveal information about his or her health status”.
Conclusions
IESs are a promising technology for improving health outcomes and making health care more effective. The enhanced control over the use and uptake of drugs might even help in the fight against pressing societal problems such as antibiotic resistance. However, IESs also raise ethical and legal challenges. On the ethical side, there are key challenges for IESs relating to patients, physicians, and society more generally. Such issues should be considered at the earliest stages of the development process of such products—the goal is ethics by design—rather than after a product has been designed and tested. There are also new legal developments in the US and Europe that are relevant for IESs. For example, the US FDA has only recently proposed for public comment a framework for regulating PDURS. In the EU, a new regulatory framework on medical devices (MDR) and invitro diagnostic medical devices (IVDR) came into force on 25 May 2017. The MDR will generally apply from 26 May 2020 and the IVDR from 26 May 2022. IESs also need to comply with the applicable data privacy laws. As regulators gain more experience with IESs, it is likely (and indeed hoped for) that these pathways will change to facilitate both innovation and high standards of safety and effectiveness as well as data privacy. For IES products to be broadly accepted by society and markets, it is, in particular, of vital importance to enhance public trust. Hence, companies developing IESs and healthcare providers using such products need to gain and maintain patient trust with regard to the management and use of the collected data. Within this trajectory privacy protection, cybersecurity, accountability, transparency, explainability, fairness, and robustness are of pivotal importance.
Received: 16 January 2019; Accepted: 17 July 2019; Published online: 15 August 2019
And it's a short way from Ingestible Sensors to Syringe-Injectable Electronics
https://www.nanowerk.com/spotlight/spotid=55185.php
May 19, 2020
Injecting biomedical electronics for monitoring and therapy of body organs
(Nanowerk Spotlight) Implantable electronic devices range from sensors, gastric and cardiac pacemakers, cardioverter defibrillators, to deep brain, nerve, and bone stimulators. These devices are interfaced with the human body to extract precise medical data and to interfere with tissue function by providing electrical stimuli. Long-term implants present specific engineering challenges, including low energy consumption and stable performance.
Furthermore, most electronic materials have poor bio- and cytocompatibility, resulting in immune reactions and infections.
A recent review in Advanced Materials ("Injectable Biomedical Devices for Sensing and Stimulating Internal Body Organs") summarizes the latest developments in materials, designs, and manufacturing techniques in the field of injectable biomedical devices, highlighting unique applications and demonstrations of viable clinical tools that were applied in various internal organs.
They also summarize the possible concerns and suggested solutions related to the development of injectable biomedical electronics and stress that more work is needed to conduct the safety assessments associated with long term use of these devices before they can be ready for clinical use.









Help! I want to get off this planet spining out of control through space that has medicine men interfering with the blueprint of life for fun and profit! We keep drilling down on the micro level and deciding how we can manipulate processes for profit! No regard for the violation of human rights.
Whatever is transcribed into Blue Tooth Technology is also transcribed via 5G Technology...Just another way the International Mafia Death Cult is to CONSTANTLY SURVEIL the population. Have no doubt it's possible to activate genes or chemicals to be made within the body to create a SUDDEN DEATH/Euthanasia event. The FREAKS ARE DOING ALL POSSIBLE FOR THE CRAVING OF ABSOLUTE GOD-LIKE POWER OVER ALL LIFE ON THE PLANET.