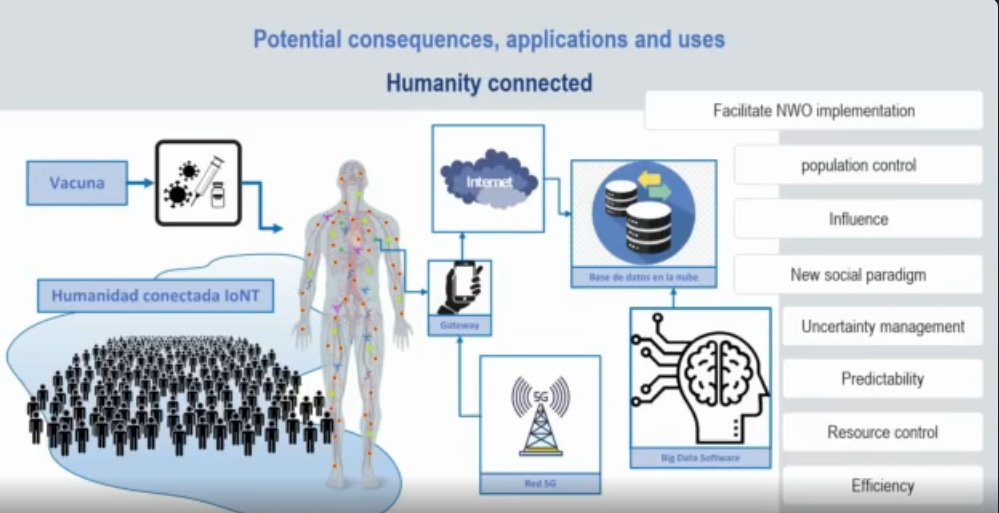
WHERE IS THE CRIMINAL INVESTIGATION? WHERE ARE THE PROSECUTORS?
https://odysee.com/@dharmabear:2/BlueTRUTH-Andree-Lealle-2022-English:4
https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-019-0542-7 Nanosensors and particles: a technology frontier with pitfalls | Journal of Nanobiotechnology | Full Text (biomedcentral.com)
As the amount of engineered nanoparticles that enter our environment is currently exponentially increasing, much tighter attention needs to be paid to assessing their health risk. This is urgent as the asbestos story told us important lessons how financial interests arising from a rapid build up of a flourishing industry has blocked and is still preventing a worldwide ban on asbestos, nearly 100 years after the first health risks were reported.
After the annual production of engineered nanoparticles increased from less than 60 tons worldwide in 2005 to an estimated 1000-fold today, many of them are released into our environment and make their way back into the food chain. This already leads to significant daily uptakes in plants, animals and humans. The asbestos story told us important lessons how financial interests arising from a rapid build up of a flourishing industry around a material with remarkable technical properties. Economic interests have blocked and are still preventing a worldwide ban on asbestos, even today. This is happening despite early warnings of severe health risks that go back to the 1920s, and broad recognition that asbestos causes cancer in the 1960s. In Switzerland, for example, the Schweizerische Unfallversicherungsanstalt (SUVA) recognized asbestosis as profession-related disease in 1939, yet the usage of asbestos was banned in Switzerland only in 1995. This illustrates that major investments into studying the health risks of nanoparticles and sensors that enter the environment or the human body in high quantities, most prominently via the food chain or the air are urgently needed.
https://www.intechopen.com/chapters/70500 Challenges for Assessing Toxicity of Nanomaterials | IntechOpen
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7379444/ Nanotoxicity: a challenge for future medicine - PMC (nih.gov)
Results and conclusion
Most authors state “the only valid technology will be nanotechnology in the next era”; however, there is no consensus on the impact of this technology on humankind, environment and ecological balance. Studies dealing with the toxic effect of nanomaterials on human health have also varied with developing technology. Nanotoxicology studies such as in vivo-like on 3D human organs, cells, advanced genetic studies, and -omic approaches begin to replace conventional methods. Nanotoxicity and adverse effects of nanomaterials in exposed producers, industry workers, and patients make nanomaterials a double-edged sword for future medicine. In order to control and tackle related risks, regulation and legislations should be implemented, and researchers have to conduct joint multidisciplinary studies in various fields of medical sciences, nanotechnology, nanomedicine, and biomedical engineering.
3.2.2. Carbon-based
Typical carbon-based nanomaterial is carbon nanotubes.
3.2.4. Quantum dots
Quantum dots are engineered nanoscale crystals that can transport electrons and they can covert a spectrum of light into different colors.
5. Nanotoxicity
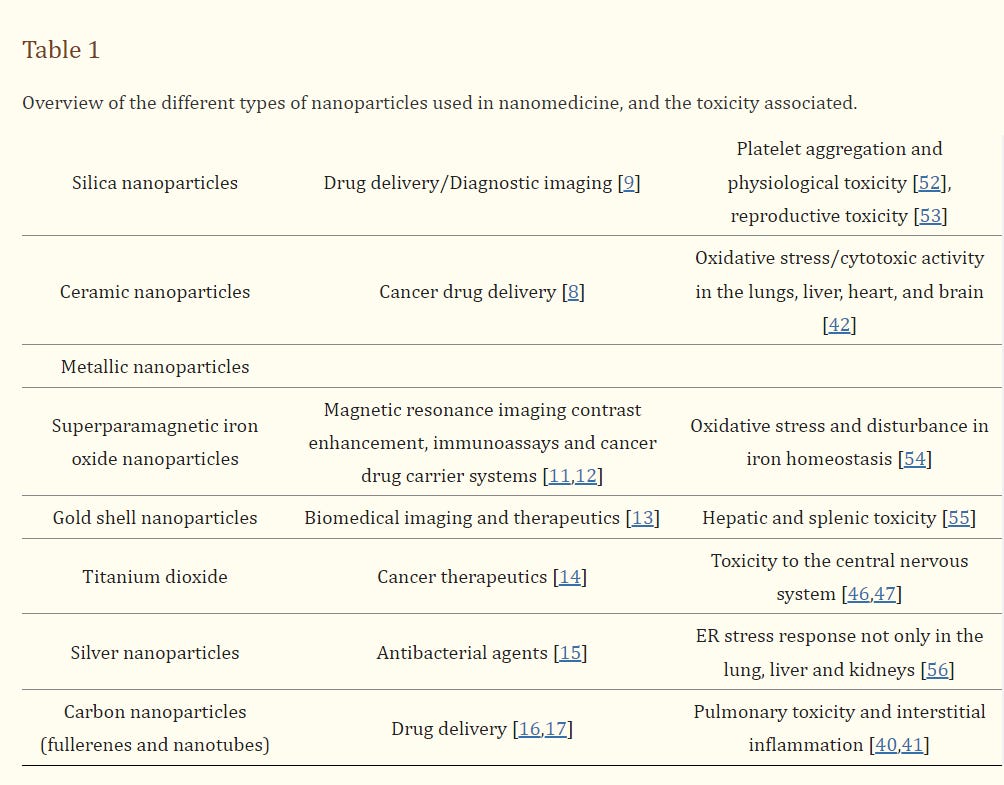
The painful experience of human beings with carcinogenic products such as tobacco products and asbestos, which they initially thought innocent, also caused a question mark for NPs. Because some NPs have long, thin, fibrous structure asbestos-like, show fibrogenic and toxic effects
“Can nanoparticles be asbestos of the future?” caused the question to be asked.
9. Toxicity mechanisms of nanoparticles
Mechanical effects due to the physicochemical properties of nanoparticles cause toxicity. The basic mechanism of toxic effect formation is reactive oxygen species (ROS) formation, either directly or indirectly.
11. Concerns, future aspects and concluding remarks
Since most authors state that “the only valid technology will be nanotechnology in the next era”, there is no consensus on the impact of this technology on humankind, environment and ecological balance.
Nanotoxicity and adverse effects of nanomaterials in exposed producers, industry workers, and patients make nanomaterials a double-edged sword for future medicine.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5304638/ Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death - PMC (nih.gov)
Both in vivo and in vitro studies have shown that nanoparticles are closely associated with toxicity by increasing intracellular reactive oxygen species (ROS) levels and/or the levels of pro-inflammatory mediators. The homeostatic redox state of the host becomes disrupted upon ROS induction by nanoparticles. Nanoparticles are also known to up-regulate the transcription of various pro-inflammatory genes, including tumor necrosis factor-α and IL (interleukins)-1, IL-6 and IL-8, by activating nuclear factor-kappa B (NF-κB) signaling. These sequential molecular and cellular events are known to cause oxidative stress, followed by severe cellular genotoxicity and then programmed cell death.
2. Toxicity of Nanoparticles
Despite the gaining popularity of nanotechnology in the field of medicine, their applications have been restricted due to their potential toxicity and long-term secondary adverse effects. (?????????)
One possible way to prevent oxidative stress-mediated nanotoxicity is the introduction of ascorbic acid upon nanoparticle exposure.
Ascorbic acid, also known as vitamin C, is an antioxidant capable of scavenging free radicals. By introducing ascorbic acid into AgNP-treated acute myeloid leukemia cells there is a complete decrease in ROS production in the cells. Concomitantly, ascorbic acid also led to a decrease in AgNP-induced mitochondria damage, apoptosis and DNA damage, reducing the toxic effects induced by AgNPs. Similar mitigation of ROS generation and glutathione depletion were also observed when ascorbic acid was added to human lung epithelial (A549) cells treated with nickel ferrite nanoparticles (26 nm in diameter). An in vivo Drosophila melanogaster study has also shown a decrease in nanotoxicity when ascorbic acid was supplemented in the diet of Drosophila exposed to AgNPs. In vivo studies with rats have further revealed that acute oxidative stress and inflammation induced by ZnO nanoparticles (of particle size 21 nm) were alleviated when 1% aqueous ascorbic acid was given as drinking water.
Hence, the administration of ascorbic acid after nanomaterial exposure is a feasible strategy to overcome nanotoxicity, both in vitro and in vitro.
Quercetin, a naturally occurring flavonoid in many plants and food, is an anti-oxidant having free radical scavenging ability. Quercetin has been found to reduce Fe2O3 nanoparticles-induced oxidative injury and inflammation by increasing Bad phosphorylation and Nrf2 translocation through PI3-K/Akt dependent pathways. In vivo studies have also revealed that TiO2 NPs induced liver and kidney oxidative stress can be circumvented by treatment with quercetin.
https://drdavidnixon.com/1/en/topic/video Video - Dr David Nixon - 1
https://mainichi.jp/english/articles/20230307/p2a/00m/0na/020000c












Burdock root tea is a whole food medicine, that kept me safe during a mystery sickness I had late 2019 -it contains quercitin, inulin (helps keep blood sugar up, roasted root is a bit sweeter), and hundreds of other helpful constituents . Drank a pint of Burdock root tea daily for 2 months straight and recovered.
Grows all over the USA. Mountain Rose Herbs in Eugene OR carries it as well.
ALC-0315;2036272-55-4-德尔塔(Delta)生物试剂 (delta-f.com) ALC-0315; 2036272-55-4
"Delta supplies ALC-0315; 2036272-55-4 for scientific research only, and not for human treatment, drug development or other commercial use. If any purchaser or third party purchases our ALC-0315; 2036272-55-4 for treatment, drug development or commercial use, the purchaser or third party will assume all legal responsibility and be held liable. ALC-0315; 2036272-55-4 is in stock and can be ordered with confidence."
www.delta-f.com/details/847570 Delta supplies ALC-0159(CAS:1849616-42-7) only for scientific research and cannot be used for human treatment, drug development or other commercial purposes. ALC-0159(CAS:1849616-42-7) is in stock and can be ordered with confidence."
In addition, both pages state that the company produces, among other things: "synthetic phospholipids, polymeric polyethylene glycol derivatives, block copolymers, magnetic nanoparticles, nano-gold and nano-gold rods, near-infrared fluorescent dyes, reactive fluorescent dyes, fluorescently labeled dextran BSA and streptavidin, protein cross-linking agents, small-molecule PEG derivatives, dot measurement chemicals, dendrimers, cyclodextrin derivatives, oncology APIs, macrocyclic ligands and other products. Top oncology APIs, macrocyclic ligands, fluorescent quantum dots, hyaluronic acid derivatives, graphene or graphene oxide, carbon nanotubes, fullerenes, silica and mesoporous silica, polymer microspheres, NIR fluorescent dyes, polystyrene microspheres, upconversion nanoparticles, MRI NMR products, fluorescent proteins and fluorescent probes, etc."
Especially noteworthy here is the fact that this company produces "graphene or graphene oxide, carbon nanotubes and fullerenes."
https://www.caymanchem.com/news/sm-102-statement SM-102 for research use only (RUO)
"Products in the RUO class, such as SM-102 (Item No. 33474), are intended for in vitro or animal (exploratory or preclinical) use only.
Safety Data Sheets must show the contents and hazards of each ingredient in the chemical product supplied. Cayman's SDS for SM-102 (Item No. 33474) accurately depicts that the mixture of chemicals in this product is 90% ethanol (a common solvent) and 10% SM-102. Although ethanol is a common solvent, it has several known serious hazards, which are discussed in Cayman's SDS. Neither the National Institute for Occupational Safety and Health (NIOSH), the Register of Toxic Effects of Chemical Substances (RTECS), nor the European Chemicals Agency's (ECHA) Inventory of Classification and Labeling list any hazards associated with SM-102."