The following studies on nanotechnology (Review of the toxicity of particles intentionally produced for nanotechnology applications, from an occupational health perspective) are not the latest. However, they show that nanotechnology is toxic, and government agencies are well aware of this, but have decided to give it the green light.
It is toxic to both humans and the environment. It's sick.
Add to that 5G, and we have a recipe for world annihilation.
Regarding 5G, listen to this podcast with Associate Professor Olle Johansson:
https://open.spotify.com/episode/2NSSCza27E6dpCYEG7RNdM 5G facts or conspiracies? --- with Professor Olle Johansson, interviewers: Frank Nilsen & Stian Nicolaysen
“We can read that 5G is dangerous and can be used as a weapon! Is it true? The phenomenon of electrical hypersensitivity ... is it just pseudoscience or can it be proven in studies? To solve our questions about 5G and radiation from wireless devices Olle Johansson helped us answer these questions and many more.”
In the podcast, they talk i.a. about:
The mobile and insurance industry are exempt from radiation liability since 1990`s;
Clinical trials done to prove electrical hypersensitivity;
What is 5G and are there any good studies to prove harm?;
Why are there so many insects dying?;
What happens when bacteria are exposed to wireless radiation;
What can we do?;
And lots more!
We've got to stop these lunatics talking about "ecology" and destroying life on this planet.
Page not found…
http://www.hse.gov.uk/aboutus/hsc/iacs/acts/watch/130105/p2annex1.pdf
Found here:
http://web.archive.org/web/20060530052044/http://www.hse.gov.uk:80/aboutus/hsc/iacs/acts/watch/130105/p2annex1.pdf A review of the toxicity of particles that are intentionally produced for use in nanotechnology applications, seen from an occupational health perspective
attached as an appendix.
2.1. Human health effects
2.1.1. Inhalation Effects on the respiratory tract
An immediate consideration of inhalation exposure to poorly soluble particles is for the consequences to the respiratory tract and lung. The broadest group of lung diseases attributable to particle exposure is pneumoconiosis. The term literally means ‘dusty lungs’; however, medically, pneumoconiosis is defined as the non-neoplastic reaction of the lungs to inhaled mineral or organic dust and resultant alteration in their structure excluding asthma, bronchitis and emphysema (Parkes, 1982). Pneumoconiosis can develop as a result of exposure to fibrous or non-fibrous particles. The severity of pneumoconiosis can range from very mild to severe. In its mildest form, it essentially represents dust accumulation, with minimal lung effects and only very minor changes in lung structure without adverse functional consequences (e.g. siderosis associated with iron exposure; stannosis associated with tin exposure). In its most severe form, fibrotic changes in the lung can lead to deficiencies in gas exchange and impaired lung function and can be fatal (e.g. silicosis associated with silica exposure; asbestosis associated with asbestos exposure). In some cases, those exposure situations eliciting the most agressive forms of pneumoconiosis have also been causally linked to the development of lung cancer e.g. asbestos and silica. In addition to lung cancer, exposure to asbestos is causally associated with the development of malignant mesothelioma. However, it is clearly not the case that severe pneumoconiosis is generally associated with progression to lung cancer – for example, there is no evidence for an increased risk of lung cancer in coal miners with fibrotic forms of pneumoconiosis. In addition to pneumoconiosis, there are other types of lung toxicity that merit consideration in terms of potential consequences of particle exposure: namely, bronchitis, emphysema and asthma. Bronchitis, a condition often associated with ‘dusty’ occupations, is inflammation of the bronchi and is characterised by increased mucous secretion in the bronchial tree. It can produce airflow obstruction. Emphysema is a condition that affects the alveolar sacs, causing a break down of the alveolar walls, resulting in fewer, larger air spaces. Emphysema has also been associated with ‘dusty’ occupations, although the main cause is cigarette smoking. Asthma is a disease in which the airways become hypersensitive and are prone to constriction, with swelling of the airway lining, leading to airflow obstruction. It is often presented as an allergic response; however, for a number of causes of asthma the mechanism(s) involved have not been clearly established.
Systemic effects
There are two aspects to consider in relation to the potential systemic effects of exposure to poorly soluble particles. The first is the ability of inhaled particles to become systemically available, leading to adverse systemic consequences.
The 8 second is the potential for materials to leach from particles contained within the lung, so that although the particle itself does not become systemically available, its leached components may.
In relation to the first aspect, inhalation exposure to poorly soluble micrometre particles is not generally associated with systemic effects of material that remains in particulate form. There are certain examples of particle exposures where systemic consequences of inhalation exposure have been proposed (for example for particulate air pollution and for silica exposure), but a causal link between these exposures leading directly to particle-induced systemic effects has not been clearly established. One possible reason for a lack of systemic toxicity could be limited systemic availability of micrometre sized particles. There are considerably more examples of inhaled particles producing systemic toxicity as a consequence of slow dissolution of the particle or leaching of its components from the lungs into the systemic circulation over a long period of time – for example, the systemic toxicity associated with cadmium or lead exposure is due to systemic availability of leachates.
2.2. Mechanistic basis for local toxicity to the lung
This section describes the current understanding for how particle exposure produces adverse effects on the lung. This understanding has been developed from studies in animals, primarily the rat. Some particles have appreciable inherent cytotoxicity towards lung cells, whereas others do not; particle shape (fibrous or non-fibrous) is another important consideration here.
3. Micrometre versus nanometre: a comparison of the toxicity of nanometre particles and micrometre particles of the same substance
This section describes the known differences in toxicity between micrometre-sized particles and nanometre-sized particles of the same material. Although the information presented in this section does not relate to novel nanoparticles, knowledge about the comparative toxicity of micrometre and nanometre particles of the same material may be useful in relation to making predictions about the potential toxicity of novel nanoparticles. In addition, some of the materials covered in this section have, or could have, applications as a consequence of their nanometre size (e.g. nanometre titanium dioxide has some cosmetic applications)
3.1. Inhalation exposure
3.1.1. Effects on the respiratory tract
As described in the previous section, the main expression of toxicity following repeated inhalation exposure to poorly soluble particles in the micrometre range is to the respiratory tract.
There is an extensive body of evidence from studies in rats, which indicates that for the same material, nanometre particles are more potent (in mass terms) than micrometre particles in inducing pulmonary toxicity.
One of the first studies to demonstrate this was that by Ferin et al (1992). (Also reported by Oberdörster et al, 1994). In this study, rats were exposed to 0 or 23 mg/m3 of titanium dioxide in either the nanometre size range (primary particle size 21 nm) or micrometre size range (250 nm). Agglomeration of the particles resulted in similar aerodynamic diameters of the two particle types; consequently, deposition patterns within the lungs were expected to be comparable for both particle sizes. Exposures were for 6 hours/day, 5 days/week for up to 12 weeks, after which rats were maintained in filtered air for up to 64 weeks. Rats were sacrificed at intervals up to 64 weeks after the start of exposure for analysis of bronchoalveolar lavage fluid (BALF) and microscopic examination of the lungs. Both particle size fractions elicited an inflammatory response in the alveoli and interstitium, as indicated by an influx of polymorphonuclear leucocytes (PMN). However, the response elicited by nanometre TiO2 was markedly greater and more persistent than that produced by micrometre TiO2. The magnitude of the alveolar inflammatory response to nanometre particles was up to 43-fold higher than that to micrometre particles and persisted up to 1 year post-exposure (64 weeks from the start of exposure), whereas the less marked inflammatory response to micrometre TiO2 had resolved almost to control levels by 41 weeks. Histopathological examination of the lungs at week 41 (29 weeks post-exposure) showed a fibrotic response in animals exposed to both particle sizes of TiO2, but again, the severity of the response was greater in animals exposed to nanometre sized particles. Type II alveolar cell hyperplasia, indicative of epithelial damage, was observed in animals exposed to nanometre TiO2 but apparently not in animals exposed to micrometre TiO2. In parallel with the differences in inflammatory and pathological response seen with the two different particle sizes, there were differences in the distribution of particles in the lung. Nanometre particles were interstitialised to a significantly greater extent than micrometre particles. Post-exposure, clearance of both micrometre and nanometre particles was impaired compared with normal clearance rates. However, on a mass basis, clearance of nanometre particles was approximately 3 times slower than that for micrometre particles. The effect on clearance of nanometre particles was not associated with volumetric overloading of the alveolar macrophages; volumetric loading of macrophages with nanometre TiO2 reached only 2.6%, which is less than the 6% volume threshold for clearance impairment proposed by Morrow (1988). In animals exposed to micrometre particles, clearance rate had returned to normal by week 41, but remained impaired in animals exposed to nanometre TiO2. Overall, therefore, this study demonstrated that nanometre particles induce a greater inflammatory response and a more marked effect on clearance than micrometre particles, on a gravimetric basis. However, when the effects on pulmonary inflammation and clearance were considered with respect to particle surface area rather than mass dose, the two particles sizes exhibited a similar degree of potency. Thus, particle surface area rather than mass dose appeared to be a critical determinant of pulmonary response. The smaller particles had a greater surface area per unit mass, and this appeared to be the basis for their greater response per unit mass. The finding that toxicity per unit mass of the same substance is enhanced as particle size decreases has subsequently been confirmed consistently across a range of exposure situations (single, short-term and long-term repeated exposure, using either intratracheal instillation or inhalation exposure) for a range of poorly soluble substances - aluminium trioxide (Oberdörster et al, 1990), carbon black (e.g. Gallagher et al, 2003; Gilmour et al, 2004; Li et al, 1996; Renwick et al, 2004), metallic cobalt (Zhang et al, 2000), metallic nickel (Zhang et al, 2003) and titanium dioxide (e.g. Borm et al, 2000; Heinrich et al, 1995; Lee et al, 1985; Oberdörster et al, 1990, 1992; Renwick et al, 2004). In addition to the potential explanatory hypothesis that toxicity is related to particle surface area, a number of other hypotheses have emerged to explain why nanometre particles should exert a greater pulmonary toxicity than micrometre particles on a mass basis. Each of these various hypotheses is discussed below. It is also worthy of note that the species differences in susceptibility to the pulmonary effects of inhaled micrometre sized particles (i.e. rat more susceptible than mouse or hamster) have been confirmed for nanometre titanium dioxide, in a repeated exposure inhalation study (Bermudez et al, 2004). 1) Particle deposition characteristics Particle deposition characteristics within the respiratory tract vary with particle size. A description of particle deposition characteristics within the respiratory tract is given in the appendix. For the same exposure in terms of mass dose, nanometre and micrometre particles distribute differently within the respiratory tract. Particles in the nanometre range are more likely to deposit in the alveoli than particles in the micrometre range. This could be a factor in the enhanced toxicity of nanometre particles (Oberdörster, 2001). However, it is also known that nanometre particles tend to agglomerate (Jefferson, 2000; Preining, 1998). As a consequence, the aerodynamic characteristics of agglomerated particles may well be similar to those of micrometre particles, with the result that in practice, there may be little difference in the deposition characteristics of nanometre and micrometre particles. This would reduce the likelihood that differences in toxicity are related to differences in deposition.
2) Particle surface area
The observation that
for the same material, nanometre sized particles are more potent inducers (on a mass basis) of pulmonary toxicity than micrometre sized particles
led to the hypothesis that particle surface area is a key determinant of toxicity (Driscoll, 1996; Oberdörster et al, 1992, 1994). Driscoll (1996) noted that for a range of insoluble, non-cytotoxic particles, pulmonary tumours observed in experimental studies in the rat were associated with lung burdens of particles with a total surface area > 2000 cm2 . Based on studies with titanium dioxide and barium sulphate, Tran et al (2000) suggested that a threshold for pulmonary inflammation could be identified at a total particle surface area of around 200-300 cm2 . They developed a model to explain how particle surface area might influence pulmonary toxicity. Central to their model is the generation of macrophage-attracting chemotactic factors as a result of particle contact with the lung lining fluid and epithelial cells. They propose that the magnitude of the chemotactic signal may be determined by particle surface area; particles with a high surface area per unit mass (nanometre particles) trigger a greater signal because of the greater particle-cell contact. This signal would lead to the recruitment of AMs and PMNs. However, with very large total surface areas of particles, the magnitude of the signal could be so strong as to disrupt the normal chemotactic gradient, thereby preventing AM migration to the mucociliary escalator, regardless of their particle burden. Impaired AM clearance would result in prolonged contact between unphagocytosed particles and the alveolar epithelium and lead to an enhanced inflammatory response. This situation would also tend to favour interstitialisation of the unphagocytosed particles. In further support of this hypothesis, Renwick et al (2004) demonstrated increased migratory activity of AMs towards the chemotaxin C5a, following intratracheal instillation of nanometre carbon black and nanometre titanium oxide in rats; equivalent mass doses of micrometre carbon black or titanium dioxide had no effect on chemotaxis. Increased chemotactic responsiveness to C5a could act to retain AMs at the site of particle deposition in the lung. Another factor that could be affected by particle surface area is the phagocytic efficiency of AMs. Here, the potential influence of particle surface area is through free radical generation; greater surface area could result in greater free radical generation, with consequences for AM function as a consequence of oxidative stress. However, although nanometre particles of carbon black and titanium dioxide have been shown to be more potent than their micrometre counterparts in impairing murine AM phagocytosis in vitro (Renwick et al, 2001), this effect was not demonstrated in vivo (Renwick et al, 2004). Further elucidation of the potential effects on AM phagocytosis of nanometre compared with micrometre particles in vivo would be useful. Another feature of particle surface area that could be relevant to the toxicity of nanometre compared with micrometre particles, relates to catalytic efficiency. The availability of a high surface area promotes the ability of a material to function as a catalyst. This is one aspect of nanoparticles that will have applications in nanotechnology, but its consequences in biological systems are not yet understood. It may be a factor in the observations that particle surface area is a key determinant of toxicity.
3) Particle surface characteristics
It has been predicted and shown experimentally for some materials, that the surface characteristics of particles in the nanometre range are markedly different from the surface characteristics of the bulk material (Jefferson, 2000; Preining, 1998). All particles adopt structures that minimise surface energy. For nanoparticles, the structures that would normally be adopted by the material in micrometre form are not necessarily the most energy efficient, because of the high ratio of surface to bulk atoms. In some cases, to achieve the most energy-efficient state, nanoparticles may adopt structures that do not exist in the larger scale. Consequently, the physicochemical properties of nanoparticles may be different from particles of the same material in the micrometre range. On this basis, it might be expected that the difference in the surface properties of nanometre particles compared with micrometre particles could be a significant factor in the differences seen in their pulmonary toxicity. Some experimental evidence for this is available. Donaldson et al (1996) measured free radical generating activity for a range of non-fibrous and fibrous particles. They proposed that the ability of particles to generate free radicals could be critical to their toxicity. They showed that free radical generating ability was greater for nanometre titanium dioxide compared with the same mass of micrometre titanium dioxide. Subsequently, Zhang et al (1998) also showed that for the same material, particles in the nanometre size range produced greater free radical generating activity than particles in the micrometre range. Oberdörster (2001) also reported on the importance of particle surface properties, based on a study using nanometre TiO2 (primary particle size 20 nm). Native TiO2 has a hydrophilic surface; however, the surface can be rendered hydrophobic by application of an appropriate surface coating, in this case a silane compound. Both uncoated and coated TiO2 was instilled into rat lungs. At 24 hours following instillation, a clear difference in the inflammatory response was seen; the hydrophobic, coated particles elicited a much lower inflammatory response than the hydrophilic particles.
4. Differences/similarities in toxicity between nanoparticles of different existing materials
4.1. Inhalation exposure
4.1.1. Effects on the respiratory tract
The importance of particle surface area in the toxicity of inhaled poorly soluble particles has been clearly established, as described in the previous section. The information presented in that section shows that for the same material, smaller particles elicit a greater pulmonary inflammatory response than do larger particles, on a mass basis. In addition, it has been shown that particle surface area can provide a unifying dose metric for a range of different insoluble particles of low cytotoxicity – regardless of the particle type, toxicity to the respiratory tract (in terms of rat lung tumour response) is related to particle surface area (Driscoll, 1996). However, two other factors have been proposed to be involved in determining the respiratory tract response to particle exposure:
1. particle surface activity – specifically, the ability of the particle surface to generate free radicals (Donaldson et al, 1996);
2. particle agglomeration/disagglomeration – a determinant of the availability of individual particles to the lung surface once the material has entered the lung (Oberdörster, 1996).
These factors may well be related to particle surface area, but they are also particle-specific and may be an important determinant of inherent toxicity towards the respiratory tract. The available data that explore how these factors may lead to differences in pulmonary toxicity between different existing nanometre materials are summarised below.
Differences in disagglomeration
As described previously,
particles in the nanometre range are unlikely to remain as singlet particles in the atmosphere for any length of time, but will tend to agglomerate (Preining, 1998).
The extent to which disagglomeration occurs once particles enter the lung may influence the subsequent toxicity. One hypothesis that has emerged from studies with different particles in the nanometre size range is that differences in pulmonary responses could be related to the extent of disagglomeration (e.g. Oberdörster et al, 1992; Takenaka et al, 1986). The first evidence for this hypothesis emerged from a study using nanometre particles of titanium dioxide and carbon black (Oberdörster et al, 1992). In this study male rats (4 per group) were instilled with saline or 500-1000 µg rutile TiO2 (particle diameter 12 or 250 nm), anatase TiO2 (particle diameter 20 or 250 nm) or carbon black (particle diameter 20 nm). For all types of particle in the nanometre size range, administration was as aggregates of particles. Assessment of inflammatory changes and lung dosimetry were determined 24 hours post-instillation. The inflammatory response seen in the alveolar space was dependent on the location of particles within the lung. The highest inflammatory response was seen for carbon black; however, carbon black particles showed relatively little translocation to the pulmonary interstitium. In contrast, the two highest doses of nanometre titanium dioxide elicited relatively mild inflammatory responses; however, these particles also showed the greatest degree of translocation to the pulmonary interstitium. The authors suggested that one explanation for this result could be a difference in the disagglomeration rate of the two particle types. If TiO2 particles disagglomerate more rapidly into singlet particles than carbon black particles of the same primary diameter, AM clearance would be slower for TiO2 than for carbon black. The resultant increase in contact time between the particles and the epithelial surface would enhance interstitial uptake. In support of this, other studies have shown that not all nanometre particles undergo interstitialisation to the same extent. For example, 15-30 nm gold particles were rapidly phagocytosed by alveolar macrophages, with limited interstitialisation (Patrick and Stirling, 1988). In comparison, 20 nm particles of Al2O3 were interstitialised similarly to 30 nm particles of TiO2 (Ferin et al, 1990). Overall, some studies point to the possibility of an effect of disagglomeration on the behaviour of nanoparticles. However, there is no experimental evidence to show whether or not such an effect actually occurs in practice. Nevertheless, the possibility remains that disagglomeration is an important feature of particle toxicity to the lung.
4.1.2. Systemic effects
The only relevant information in relation to comparisons of the systemic toxicity potential of different nanometre particles comes from studies that have looked at their systemic distribution (see appendix for details of individual studies). The studies that have been performed to date have given conflicting results in terms of the extent of extrapulmonary translocation following inhalation exposure. For example, significant translocation to the liver and other organs has been reported by a number of authors, for a variety of particle types, in animals and human volunteers (Nemmar et al, 2001, 2002; Oberdörster et al, 2002; Takenaka et al, 2001); whereas other authors have found no significant systemic distribution of nanoparticles (Brown et al, 2002; Kreyling et al, 2002, 2004; Semmler et al, 2004). One possible explanation for the observed differences in systemic translocation may be related to the exposure conditions, chemical composition and particle size of the different types of nanometre particle used in these studies. Kreyling et al (2002) proposed two hypotheses that may explain differences in the results they saw for iridium nanoparticles (negligible systemic translocation) and those reported by Oberdörster et al (2002) (significant systemic translocation of 13C nanoparticles). The first hypothesis related to possible differences in disagglomeration. If 13C nanoparticles dissagglomerate in the lung more to a greater extent than 192Ir nanoparticles, this could enhance the direct passage of singlet particles across the lung epithelium. The second hypothesis concerned
the tendency of particles to bind to high molecular weight proteins,
which would then influence their subsequent fate. Either hypothesis could explain the observed differences. Clearly there remain uncertainties surrounding the systemic availability of nanometre particles in general, and how different nanoparticles might behave in terms of their systemic distribution following inhalation exposure. If there are differences in systemic availability according to individual particle characteristics, then it would be also predicted that this could have consequences for the expression of any systemic toxicity; different materials in nanometre form could have different systemic effects.
5.1. Carbon nanotubes
A description of carbon nanotubes is given in section
1.1.1. 5.1.1. Single exposure toxicity to the respiratory tract
There are no studies using the inhalation route of exposure. The only information comes from three intratracheal instillation studies in rodents. These studies were performed for preliminary screening purposes only, and each included a comparison with reference particulate materials. By their nature, as screening studies, they provide extremely limited information in terms of the likely health effects of exposure to carbon nanotubes (CNT) in the occupational setting. The first of the studies investigated the pulmonary toxicity of single-walled CNT (SWCNT) soot in the rat (Warheit et al, 2004). The soot consisted of SWCNT agglomerates (~30 nm diameter; 50-60% by weight), amorphous carbon (30-40%), nickel (5%) and cobalt (5%). Male rats (numbers not specified) were exposed to 1 or 5 mg/kg SWCNT by intratracheal instillation. Additional groups of control male rats (numbers not specified) were similarly exposed to 1 or 5 mg/kg of the following particles: a graphite/catalyst mixture (graphite particle size 3-10 µm; cobalt and nickel particle size 2-3 µm; metal content the same as for SWCNT); crystalline silica (Mil-U-Sil 5; positive control; particle size 1-3 µm); and carbonyl iron particles (negative control; particle size 0.8-3 µm). Pulmonary toxicity was assessed by analysis of bronchoalveolar lavage fluid (BALF) for indicators of cell damage and inflammation (lactate dehydrogenase (LDH), alkaline phosphatase (ALP), protein concentration and neutrophil numbers (PMN)); by investigation of alveolar macrophage (AM) chemotaxis; measurement of pulmonary cell (tracheobronchial and lung parenchymal) proliferation; and by histopathological examination of the lungs. All examinations were performed at 24 hours, 1 week, 1 month and 3 months post-exposure.
Graphite-exposed animals were examined only for histopathological and cell proliferation changes. Mortality (15%, actual numbers not stated) was seen within 24 hours of instillation of 5 mg/kg SWCNT. Cause of death was suffocation due to blockage of the upper airways by agglomerated SWCNT.
This is most likely to be an artefact of the dosing method, and not relevant to inhalation exposure. Surviving animals in this group showed no outward signs of toxicity and had normal weight gain. No other mortalities occurred. An immediate (24 hours) inflammatory response with cell damage was seen in animals exposed to 5 mg/kg SWCNT, evidenced by increases in PMN, LDH and protein. This response was transient, and values were similar to control levels at all other time points. No treatment-related effects were seen at 1 mg/kg SWCNT. Chemotaxis was unaffected by SWCNT exposure. Histopathological examination of the lungs of rats exposed to both dose levels of SWCNT showed multifocal granulomas, distributed diffusely and randomly in the lung. The appearance of the granulomas was not dose-related (although the lack of a dose-response could be related to the non-uniform dosing pattern associated with the exposure route) and was first observed at 1 week post-exposure. There was no apparent progression of these lesions with time. Some SWCNT agglomerates that deposited in the airways were also surrounded by granulomas, an unusual response to see outside the alveoli. A non-statistically significant increase in tracheobronchial cell proliferation rate was seen at 24 hours in animals exposed to 5 mg/kg SWCNT. Lung parenchymal cell turnover was unaffected by SWCNT exposure. In comparison, exposure to 1 and 5 mg/kg silica produced an immediate and sustained inflammatory lung response, with evidence of sustained cell damage also seen at 5 mg/kg. Transient increases in BALF ALP values (indicating toxicity to the surfactant secreting cells) were seen at both dose levels and impairment of AM chemotaxis was seen at 5 mg/kg. Histopathological examination of silica-exposed animals revealed a dose-related inflammatory response, characterised by neutrophils and foamy (lipid containing) AM accumulation, with lung tissue thickening, indicative of progression to fibrosis. There was an increase in tracheobronchial cell proliferation rate at 1 and 5 mg/kg silica (not statistically significant). Lung parenchymal cell turnover was statistically significantly increased in animals exposed to 5 mg/kg silica at 24 hours and 1 month. The only finding in animals exposed to carbonyl iron was an immediate (24 hours), transient influx of PMNs. There were no other changes in BALF parameters, and neither carbonyl iron exposure nor graphite exposure produced any adverse histopathological findings or changes in cell proliferation.
Overall, this preliminary screening study indicates an immediate, transient inflammatory lung response to instilled SWCNT.
Histopathologically, SWCNT produced granulomas within 1 week post-exposure, although unusually, this was in the absence of sustained inflammation or cell damage. The pulmonary response to SWCNT was closer to (but not as pronounced as) that produced by silica than by carbonyl iron or graphite,
which suggests that SWCNT has some inherent cytotoxicity.
The second screening study investigated the toxicity of three different types of SWCNT, produced by different methods and with different metal contents (Lam et al, 2004). Raw (RNT), purified (PNT) and nickel-containing (CNT) nanotubes were administered in a single dose to mice by intratracheal instillation. RNT contained 27% iron (by weight). PNT was treated to remove residual metal and contained 2% iron. CNT contained 26% nickel and 5% yttrium. Other metals in RNT and CNT were present at <1%. Carbon black and silica (Mil-U-Sil-5), administered at the same dose levels as SWCNT, were included as reference dusts and mouse serum was used as the vehicle control. The particle size of the nanotubes and of the reference dusts was not stated. Nanotubes were sonicated prior to administration to reduce agglomeration. Groups of male mice were intratracheally instilled with 0.1 or 0.5 mg of each particle type (approximately 3.3 or 16.7 mg/kg respectively, for a 30 g mouse). Based on the assumption that a mouse breathes 30 ml air per minute, and that 40% of respirable nanoparticles deposit in the pulmonary region, instillation of 0.5 mg SWCNT equates to an inhalation exposure of 5 mg/m3 , 8 hours/day for 17 days. Animals were sacrificed at 7 or 90 days post-instillation and the lungs were examined histopathologically. Body weight was measured in animals sacrificed at 90 days. Deaths (5/9) occurred within 4-7 days post-instillation in animals treated with 0.5 mg CNT. Animals in this group were lethargic and inactive and showed a bodyweight loss of about 27% within the first week post-exposure. Survivors showed no clinical signs after one week, and gained weight. No deaths, clinical signs or bodyweight losses occurred at 0.1 mg CNT or in any other treatment group or controls. Exposure to 0.5 mg RNT produced some clinical signs (some inactivity, hypothermia, piloerection and occasional shivering), 8-12 hours following treatment, but animals were normal after this time and no weight loss occurred. No clinical signs were seen with 0.1 mg RNT, or in animals treated with PNT, carbon black or silica. Gross examination of the lungs at 90 days post-exposure showed abnormalities in some animals exposed to 0.5 mg CNT, PNT or RNT. Histopathological examination showed granulomas, often in the interstitium. There was some evidence of necrosis and interstitial and peribronchial inflammation. The lesions seen at 90 days were generally more pronounced than those seen at 7 days. Granuloma formation and inflammation was seen in some animals exposed to 0.1 mg RNT and PNT but not 0.1 mg CNT; the lesions were less severe than seen with the higher dose. Exposure to carbon black did not produce any inflammatory or granulomatous changes in the lungs. Exposure to 0.1 mg silica produced an inflammatory response in a single animal sacrificed at 90 days; 0.5 mg silica produced a mild to moderate inflammatory response in the alveoli and interstitium at 7 and 90 days and one mouse showed a slight granulomatous response at 7 days.
Overall, this study demonstrates a toxicity response to three different types of intratracheally instilled SWCNT in the mouse lung.
Quantitative differences in response were seen between the three SWCNT materials. One form of SWCNT produced mortality; this suggests a specific effect of the particular material, perhaps associated with its metal content and the dosing method, rather than a generic effect of SWCNT. However,
lung damage (granuloma formation in the pulmonary interstitium, progressing to necrotic damage in some cases) was seen with all three SWCNT materials, regardless of metal content.
This, together with the results of the screening study by Warheit et al (2004) in which granuloma formation was observed, suggests that granuloma formation is associated with the nanotubes themselves. The pulmonary response to SWCNT was closer to that produced by the same mass dose of silica rather than carbon black, again suggesting inherent cytotoxicity of SWCNT. In an earlier study, that was very briefly reported, guinea pigs were administered a single intratracheal dose of 25 mg of soot containing CNT (Huczko et al, 2001). The composition of the CNT material was not further defined, other than it was synthesised using a Co/Ni catalyst, and therefore is likely to have Co and Ni as impurities. It also appears that the material was likely to contain a mixture of single and multi-walled CNTs. Control animals were administered soot without CNTs. Four weeks after administration, the animals were subject to pulmonary function tests (measurement of tidal volume, respiratory frequency and lung resistance) and BALF analysis. No differences between CNT-exposed animals and controls were found in any of the measured parameters.
5.1.3. Additional information
One study has investigated the toxicity of SWCNT in cultured human epidermal keratinocytes (HaCaT) (Shvedova et al, 2003). Cells were exposed to SWCNT (30% iron content by mass; iron valency not stated) for up to 18 hours.
Exposure produced clear indications of hydroxyl (·OH) radical production with associated oxidative damage (lipid peroxidation, antioxidant depletion), and a reduction in cell viability.
Addition of a metal chelator suppressed ·OH generation and improved cell viability.
Ultrastructural cell changes were seen, including changes to cytoplasmic organelles and disruption of the monolayer structure. These observations are consistent with the ability of ferrous iron (Fe2+) to catalyse hydroxyl radical generation from hydrogen peroxide. However, knowledge about the valency of the iron in the SWCNT material, and on its availability to catalyse any such reactions within the skin in vivo is required before any reliable conclusions can be drawn in relation to the potential in vivo dermal toxicity of SWCNT. Derivatives of SWCNT have been shown to cross cell membranes, and in some cases, to enter the nuclei of human and mouse fibroblasts and human keratinocytes (Pantarotto et al, 2004). The study was performed to determine the potential for carbon nanotubes to deliver biologically active molecules into cells, for therapeutic applications. Carbon nanotubes (1 nm diameter, 0.1-3 µm length) were labelled either with fluorescein isothiocyanate (FITC), or with an FITC-labelled peptide.
These SWCNT derivatives were very water soluble and in the dilutions used in the test system, did not aggregate.
The FITC-labelled SWCNT readily crossed the cell membrane and was located mainly in the cytoplasm; the peptide-SWCNT conjugate reached the cell nucleus. Fluorescein alone and FITC-peptide did not cross the cell membrane in the same test system. The mechanism whereby the SWCNT derivatives entered the cells was not elucidated, but was shown not to be via endocytosis. However, the results from these very specialised SWCNT derivatives are unlikely to be relevant to exposures to SWCNTs themselves in the occupational setting.
5.1.4. Summary of health effects of carbon nanotubes
There is a paucity of information about the potential health effects of exposure to carbon nanotubes. There are no studies that utilise the inhalation route of exposure and no studies that investigate the effects of repeated exposure. The only information is from single-exposure, screening-type studies. In the three available studies that investigate potential toxicity to the rodent respiratory tract, it is apparent that single intratracheal exposure to SWCNT produces an immediate but transient inflammatory response, with subsequent lung pathology characterised by granuloma formation. The granulomatous response was seen for different SWCNT types, produced by different processes and with different metal compositions, suggesting that this is a generic lung response to carbon nanotubes. In the two studies that compared SWCNT with cytotoxic and non-cytotoxic reference dusts, the pulmonary responses to SWCNT were closer to those induced by silica (cytotoxic) than by either graphite or carbon black (non-cytotoxic). No effect was seen on pulmonary function parameters measured four weeks after exposure in one study. The limited information suggests that carbon nanotubes do not have irritancy potential to the skin or eyes. It is noteworthy that CNT produced now or in the future may fall under the definition of a ‘countable’ fibre for regulatory purposes (length > 5 µm, diameter < 3 µm, aspect ratio > 3:1 (HSE, 1996)). Given this, the toxicological concerns associated with such fibres, influenced by fibre properties such as chemical composition, surface properties and durability, must also be considered in relation to CNT.
5.2. Fullerenes
As with carbon nanotubes, there is a dearth of information about the potential toxicity of fullerenes. There is no useful information from standard toxicity studies. The limited information that is available is summarised below. In a very briefly reported study, fullerene soot, (C60 content between 0 and 15% by weight) was tested for its potential to cause skin reactions in 30 volunteers (Huczko et al, 1999). A soot suspension in water was placed in contact with the skin; the exposure time was unclear. No skin reactions were observed when assessed after 96 hours. In the same study, soot samples (0-15% C60 by mass) were instilled into the eye of 4 rabbits (0.2 ml fullerene suspension in water). No abnormality was observed at 24, 48 or 72 hours. Overall, although limited, these data suggest that fullerenes are not irritating to the skin or eye.
Fullerenes have been shown to have oxidising properties and to catalyse the production of singlet oxygen following photoexcitation.
Consequently, some concerns have been raised about their carcinogenic potential.
In a study to investigate potential tumour-promoting activity in the mouse skin (Nelson et al, 1993), groups of at least 3 female mice were treated with a single dose of 200 µg fullerene (C60:C70 ratio ~6:1; saturated solution in benzene) or 5 µg TPA (12-O-tetradecanoyl-phorbol-13-acetate; a tumour promoter) in acetone; control mice were treated with benzene or acetone only. Mice were sacrificed 0-72 hours post-exposure and the treated skin removed for analysis of ornithine decarboxylase (ODC) activity (associated with tumour promotion in mouse skin) and DNA synthesis. Fullerene produced a slight increase in ODC activity at 6 hours post-exposure, but did not increase DNA synthesis. Fullerene treatment was reported to produce only mild effects on the skin, but no further details were provided. TPA produced a marked increase in ODC activity and DNA synthesis and marked hyperplasia. These data suggest that fullerenes do not have any significant tumour promoting activity nor produce significant local skin effects following single dermal exposure. In the same study, the effects of repeated dermal exposure to fullerenes was also investigated. Mice pre-treated with DMBA and subsequently exposed to 200 µg fullerene twice weekly for 24 weeks did not develop skin tumours, whereas mice similarly treated with 5 µg TPA did. Fullerene treatment did not produce any bodyweight changes, nor any pathological changes such as neoplasia or dysplasia in the treated skin. Overall, however, this study is too limited to allow any conclusions to be drawn about the potential carcinogenicity of fullerenes. The mutagenic potential of fullerene C60 has been assessed in a non-standard study in bacterial cells (Sera et al, 1996). C60 in polyvinylpyrrolidone induced mutations in Salmonella strains TA102, TA104 and YG3003 (a repair enzyme-deficient mutant of T102) in the presence of rat liver S9 only when it was irradiated for 20 minutes by visible light. Further investigations suggested that
the mechanism for DNA damage was the generation of singlet oxygen from C60 following irradiation, which led to lipid peroxidation, the production of radicals and oxidative DNA damage.
Kamat et al (1998) investigated the potential for fullerene to induce oxidative damage following photoexcitation, using rat liver microsomes as a model biological membrane system.
C60 (as a cyclodextrin-C60 complex) was incorporated into rat liver microsomes, which were then exposed to UV or visible light.
Lipid peroxidation and other oxidative damage was observed, primarily due to the production of singlet oxygen. However, given the non-standard and experimental nature of this in vitro model, the results cannot be reliably extrapolated to the in vivo situation. Another in vitro study investigated the effects of fullerene (C60; >99% pure) and raw soot from fullerene production on bovine macrophages and macrophage-like cells (Baierl et al, 1996). Cells were incubated for up to 48 hours with C60, raw soot (RS) or DQ12 quartz as a positive control. Markers of cell damage (lactate dehydrogenase (LDH)), lysosomal damage (N-acetyl-β-D-glucosaminidase (NAG)), generation of reactive oxygen species (H2O2 and O2 - ) and markers of chemotactic activity were evaluated. Neither C60 nor RS produced any significant cytotoxicity nor lysosomal damage even up to 48 hours incubation. Both particle types elicited chemotactic activity after 48 hours, although that generated by C60 was minimal. C60 also produced very little reactive oxygen species, whereas RS produced a more marked effect; however, the nature of the reactive species could not be determined.
5.2.1. Summary of toxicity of fullerenes
There are no standard toxicological studies with fullerenes and the very limited information that is available comes from non-standard studies. There is no information on the potential consequences of single or repeated inhalation exposure, neither in terms of toxicity to the respiratory tract, nor systemically. In relation to skin exposure, all the available studies have shortcomings either in their design and/or their reporting. The only reliable conclusion that can be drawn is that fullerenes appear not to be locally irritating to the skin; similarly, there was no evidence for eye irritation potential. A few in vitro studies are available. These focus particularly on the potential for fullerenes to produce oxidative damage in various test systems. However, given the non-standard nature of these in vitro systems, no reliable conclusions can be drawn from them. Overall, therefore, there is no reliable, relevant information on the potential toxicological consequences of inhalation exposure to fullerenes, and extremely limited information on the effects of dermal exposure.
From the CDC website:
https://stacks.cdc.gov/view/cdc/10850/cdc_10850_DS1.pdf Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos
Murray et al. Particle and Fibre Toxicology 2012, 9:10 http://www.particleandfibretoxicology.com/content/9/1/10
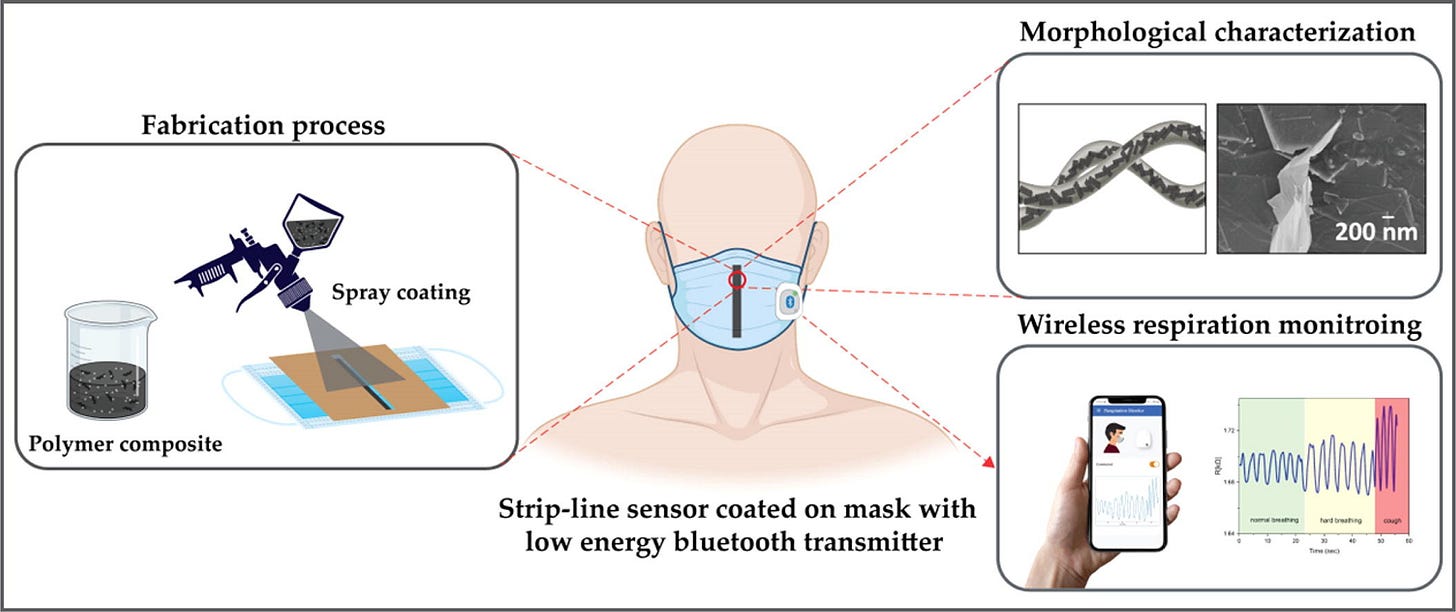
https://www.sciencedirect.com/science/article/pii/S0264127523003854 Wearable Graphene-based smart face mask for Real-Time human respiration monitoring - ScienceDirect
https://www.youtube.com/watch?v=zB4JYY64wLk Health Canada issues advisory over masks containing graphene, urges recall - YouTube
TOO SOON TO SAY IF THE MASKS ARE DANGEROUS
It’s not clear how big the masks’ graphene particles are, and every company manufacturing nanomaterials does it differently, said Nho.
However, while he cautioned that he’s not an expert on graphene in particular, he said he believes it tends to fall just above the size threshold he mentioned, at about 25 micrometres, meaning the lungs may be able to clear it out.
It needs to be studied carefully, he said, especially as this material is also sometimes treated as antiviral and therefore as a health protection.
“Used properly, it can be a benefit for public health,” he said. “But the downside is that again, if there's no proper regulation on graphene or graphene oxide, there's some studies… that graphene oxide can potentially kill lung cells.”
When told of Quebec workers’ reported symptoms, he said he hopes more in-depth studies are done to determine what other factors could have played in, and whether the masks are definitely to blame for the symptoms.
“If there's correlation, it’s really alarming,” he said. “It's really a concern.”
Elderly people above about 65 are less efficient at this as their lung function deteriorates, so they’re “more vulnerable to lung injury” and need to be especially careful about what they breathe in, Nho said.
Small children can also suffer disproportionately.
“If the graphene oxide is highly concentrated, and if it's somehow not fixed [securely on the mask], the effect may be more detrimental,” he said.
“You just directly inhale, and then the amount of nanoparticle may be very high.”









Sounds like AI written fake research.
Hopefully not a soul is receiving any grant ( tax payer ) money.
Hi, what you write is very good, if you could make summaries of important points at the top and the full versions below I think more people will grasp the gravity of these posts thanks.