NANOTECHNOLOGY IS TOXIC
Why isn't there a decent list of adverse reactions for C-19 injections?
Sorry, the pictures used are graphic, but this is the reality from the research papers.
WHY PEOPLE GET INJURED - IN COVID AND AFTER THESE INJECTIONS -AND DIE IS PRETTY SIMPLE
NANOTECHNOLOGY IS TOXIC
The technology used had been tested for years. Its negative effects were known for years.
Why wasn't there a decent list of adverse reactions after C-19 injections?
For example:
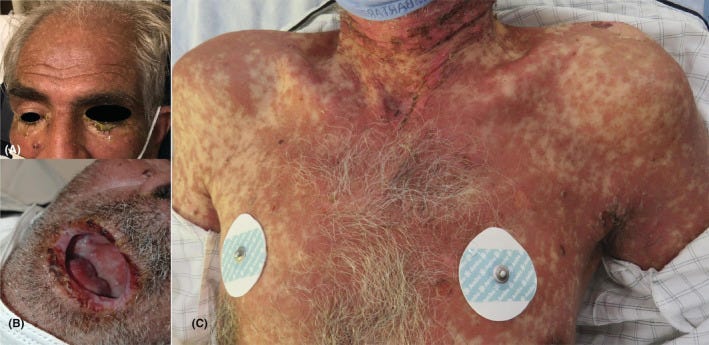
Stevens-Johnson syndrome/toxic epidermal necrolysis is the most dreaded adverse cutaneous drug reaction and is potentially fatal, if not promptly managed.
The following is a case of drug-induced toxic epidermal necrolysis in a patient with metastatic squamous cell carcinoma of the man genitals.
Toxic epidermal necrolysis manifested by erosions involving the face, conjunctival congestion, chemosis and crusting over the lips, and involvement of the trunk with sheet-like detachment of the skin.
But what drug was used?
Pembrolizumab (sold under the brand name Keytruda), BASED ON NANOPARTICLES.
Pembrolizumab was discontinued for adverse reactions.
This story is here: Pembrolizumab-induced toxic epidermal necrolysis: A rare cause of severe adverse drug reaction - https://ijdvl.com/pembrolizumab-induced-toxic-epidermal-necrolysis-a-rare-cause-of-severe-adverse-drug-reaction/ Indian Journal of Dermatology, Venereology and Leprology (ijdvl.com)
https://www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-5-10-2017 Serious AEs occurred in 41% of the patients in the pembrolizumab plus PC arm compared with 28% in the PC alone arm. The most common AEs (all grades) in the pembrolizumab + PC arm were fatigue (71%), nausea (68%), and constipation (51%). The most common grade 3-4 adverse reactions were fatigue (3.4%), dyspnea (3.4%), nausea (1.7%), vomiting (1.7%), diarrhea (1.7%), and rash (1.7%). The most common adverse reaction resulting in discontinuation of pembrolizumab (≥ 2%) was acute kidney injury (3.4%).
https://medsafe.govt.nz/Consumers/CMI/k/Keytruda.pdf KEYTRUDA can cause harm or death to your unborn baby. Do not breast-feed while taking KEYTRUDA. When you get KEYTRUDA, you can have some serious side effects. These side effects can sometimes become life-threatening and can lead to death. These side effects may happen anytime during treatment or even after your treatment has ended. You may experience more than one side effect at the same time. If you have any of the following symptoms, call or see your doctor right away. • Signs and symptoms of lung problems − shortness of breath − chest pain − coughing • Signs and symptoms of problems with your intestines − diarrhoea or more bowel movements than usual − your stools are black, tarry, sticky or have blood or mucus − severe stomach pain or tenderness • Signs and symptoms of liver problems − nausea or vomiting − feeling less hungry − pain on the right side of your stomach − your skin looks yellow − the whites of your eyes look yellow − dark urine − you bleed or bruise more easily than normal • Signs and symptoms of kidney problems − changes in the amount or colour of your urine • Signs and symptoms of hormone gland problems (especially the thyroid, pituitary, and adrenal glands) − rapid heartbeat − weight loss − increased sweating − weight gain − hair loss − feeling cold − constipation − your voice gets deeper − muscle aches − dizziness or fainting − headaches that will not go away or unusual headache • Signs and symptoms of blood sugar problems − feeling more hungry or thirsty − needing to urinate more often − weight loss • Signs and symptoms of skin problems − rash − itching − skin blistering, peeling or sores − ulcers in mouth or in lining of nose, throat, or genital area • Signs and symptoms of problems in other organs − muscle pain or weakness − changes in eyesight − stomach area pain with nausea and vomiting (pancreatitis) − shortness of breath, irregular heartbeat, feeling tired, or chest pain (myocarditis) − confusion, fever, memory problems, or seizures (encephalitis) − swollen lymph nodes, rash or tender lumps on skin, cough, or eye pain (sarcoidosis) − pain, numbness, tingling, or weakness in the arms or legs; bladder or bowel problems including needing to urinate more frequently, urinary incontinence, difficulty urinating and constipation (myelitis) − inflammation of the blood vessels (vasculitis) − decreased function of the parathyroid gland, which may include muscle cramps or spasms, fatigue and weakness (hypoparathyroidism) − pain in the upper right part of the stomach, swelling of the liver or spleen, fatigue, itching or yellowing of the skin or whites of eyes (sclerosing cholangitis) There are possible side effects of treatment with KEYTRUDA in people who have received a transplant. Rejection of a transplanted organ. People who have had an organ transplant may have an increased risk of organ transplant rejection. Your doctor should tell you what signs and symptoms you should report and monitor you, depending on the type of organ transplant that you have had. Graft-versus-host-disease (GVHD) in people with bone marrow (stem cell) transplant that uses donor stem cells (allogeneic). GVHD may occur if you had this transplant in the past. Your doctor will monitor you for the following signs and symptoms: skin rash, liver inflammation, abdominal pain, and diarrhoea. • Signs and symptoms of infusion (IV) reactions − shortness of breath − itching or rash − dizziness − fever The following side effects have been reported in clinical trials: Very common (may affect more than 1 in 10 people) • diarrhoea, nausea • itching, rash • joint pain • back pain • feeling tired • cough • patches of skin which have lost colour • stomach pain • decreased sodium levels in the blood • low levels of thyroid hormone The most common side effects when KEYTRUDA is given alone to children are: • fever • vomiting • headache • stomach pain • decrease in number of red blood cells • cough • constipation The following side effects have been reported in more than 1 in 5 people when KEYTRUDA was given in combination with chemotherapy: • hair loss • feeling tired • diarrhoea • vomiting • rash • fever • decrease in white blood cell count • decreased appetite • joint pain • swelling of the lining of the digestive system (for example mouth, intestines) • mouth sores The most common side effects when KEYTRUDA is given in combination with axitinib are: • diarrhoea • high blood pressure • fatigue • low levels of thyroid hormone • decreased appetite • blisters or rash on the palms of your hands and soles of your feet • nausea • increase in liver enzyme levels • hoarseness • cough • constipation The most common side effects when KEYTRUDA is given in combination with lenvatinib are: • high blood pressure • diarrhoea • feeling tired • decreased appetite • low levels of thyroid hormone • nausea • vomiting • weight loss • joint pain • headache • constipation • hoarseness • urinary tract infection • stomach-area (abdominal) pain KEYTRUDA® 4 • blisters or rash on the palms of your hands and soles of your feet • rash • protein in your urine • increase in liver enzyme levels • feeling weak. Less common side effects can happen. Also, your doctor may do blood tests to check for side effects. KEYTRUDA may cause other side effects that are not listed. For more information, ask your doctor. If you have any side effect that bothers you or that does not go away, tell your doctor.
Immune-mediated adverse reactions can occur with pembrolizumab including pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis.
AND IT IS A DRUG USED TO TREAT CANCER!
Here are similar cases after c-19 INJECTION:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9538630/ Toxic epidermal necrolysis after first dose of Pfizer‐BioNTech (BNT162b2) vaccination with pharmacogenomic testing - PMC (nih.gov)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9748221/ Toxic epidermal necrosis following Sinopharm COVID‐19 vaccine (BBIBP‐CorV): A case report and literature review - PMC (nih.gov)
https://www.jaadcasereports.org/article/S2352-5126(22)00201-6/fulltext Toxic epidermal necrolysis-like linear IgA bullous dermatosis after third Moderna COVID-19 vaccine in the setting of oral terbinafine - JAAD Case Reports
https://onlinelibrary.wiley.com/doi/10.1111/ajd.13742 A case of toxic epidermal necrolysis after ChAdOx1 nCov‐19 (AZD1222) vaccination - Kherlopian - 2022 - Australasian Journal of Dermatology - Wiley Online Library
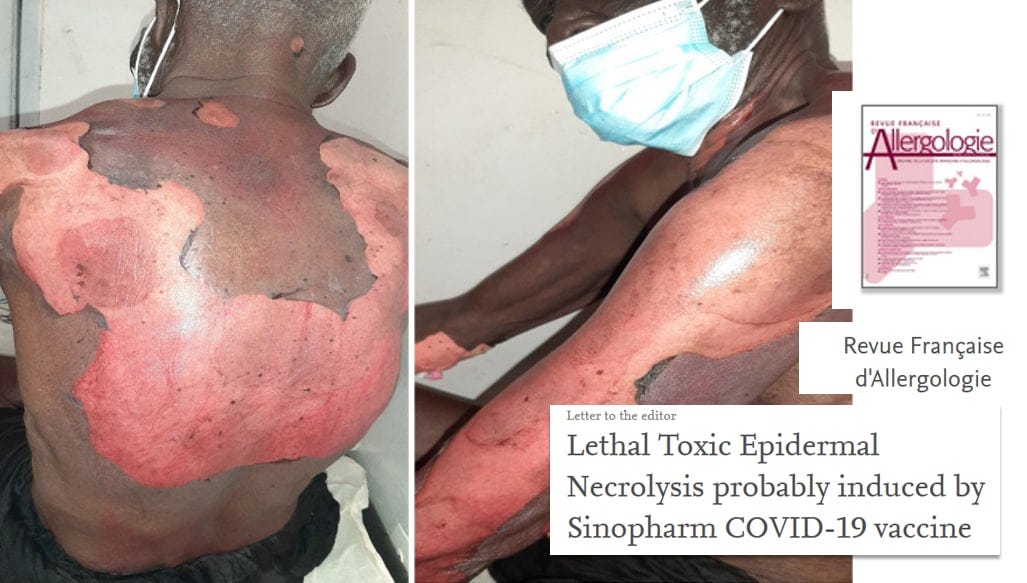
https://www.sciencedirect.com/science/article/pii/S1877032022003566?via%3Dihub#fig0005 Lethal Toxic Epidermal Necrolysis probably induced by Sinopharm COVID-19 vaccine - ScienceDirect
https://www.cureus.com/articles/68051#!/ Cureus | Toxic Epidermal Necrolysis Post COVID-19 Vaccination - First Reported Case
And yet the drug administered - COVID-19 "VACCINE" COMES WITH ALMOST NO WARNING about side effects! It is actually ADVERTISED AS SAFE AND EFFECTIVE! SAFE FOR PREGNANT AND BREASTFEEDING WOMEN!
Comparing adverse drug reactions of these two drugs: Pembrolizumab (sold under the brand name Keytruda) and COVID-19 “vaccines” (from VAERS reporting: https://medalerts.org/vaersdb/findfield.php?TABLE=ON&GROUP1=SYM&EVENTS=ON&VAX=COVID19):
Severe and Fatal Immune-Mediated Adverse Reactions:
KEYTRUDA can cause immune-mediated pneumonitis 3.4%
COVID-19 “vaccines”: Pneumonitis 638 - 0.04% and Pneumonia 7,679 - 0.52%
KEYTRUDA can cause severe or life-threatening infusion-related reactions, including hypersensitivity and anaphylaxis, which have been reported in 0.2% of 2799 patients receiving KEYTRUDA.
COVID-19 “vaccines”: Anaphylactic reaction 8,749 - 0.59%, Anaphylactic shock 1,513 - 0.1%, Hypersensitivity 9,767 - 0.66%
KEYTRUDA can cause immune-mediated colitis, which may present with diarrhea: 1.7%
COVID-19 “vaccines”: Colitis 711 - 0.05%, Colitis ischaemic 199 - 0.01%, Colitis microscopic 158 - 0.01%, Colitis ulcerative 1,050 - 0.07%, Diarrhoea 42,271 - 2.84%, Diarrhoea haemorrhagic 513 - 0.03%, Diarrhoea infectious 13 - 0%, Diarrhoea neonatal 7 - 0%
KEYTRUDA can cause immune-mediated hepatitis: 0.7%
COVID-19 “vaccines”: Hepatitis 486 - 0.03%, Hepatitis A 56 - 0%, Hepatitis acute 174 - 0.01%
KEYTRUDA can cause primary or secondary adrenal insufficiency: 0.8%
COVID-19 “vaccines”: Adrenal insufficiency 109 - 0.01%
KEYTRUDA can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitaris: 0.6%
COVID-19 “vaccines”: Hypophysitis 15 - 0%, Headache 220,156 - 14.78%, Photophobia 3,881 - 0.26%, Visual field defect 851 - 0.06%
KEYTRUDA can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism: 0.6%
COVID-19 “vaccines”: Thyroiditis 280 - 0.02%, Thyroiditis acute 76 - 0.01%, Thyroiditis chronic 3 - 0%, Thyroiditis subacute 288 - 0.02%, Hyperthyroidism 970 - 0.07%
KEYTRUDA can cause immune-mediated nephritis: 0.3%
COVID-19 “vaccines”: Nephritic syndrome15 - 0%, Nephritis 179 - 0.01%, Nephritis allergic 2 - 0%, Nephritis bacterial 1 0%
KEYTRUDA can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson syndrome, drug rash with eosinophilia and systemic symptoms, and toxic epidermal necrolysis: 1.4%
COVID-19 “vaccines”: Dermatitis 950 - 0.06%, Dermatitis acneiform 197 - 0.01%, Dermatitis allergic 815 - 0.05%, Dermatitis artefacta 1 - 0%, Dermatitis atopic 273 - 0.02%, Dermatitis bullous, 481 - 0.03%, Dermatitis contact 341 - 0.02%, Dermatitis diaper 17- 0%, Dermatitis exfoliative 21 0%, Dermatitis exfoliative generalised 135 - 0.01%, Dermatitis herpetiformis 15 - 0%, Dermatitis infected 23 0%, Dermatitis psoriasiform 54 - 0%
KEYTRUDA can cause severe or fatal cases for some of these adverse reactions: Cardiac/Vascular: Myocarditis, pericarditis, vasculitis;
COVID-19 “vaccines”: Myocarditis 16,437 - 1.1%, Myocardial infarction 5,067 - 0.34%, Pericarditis 10,890 - 0.73%, Pericardial effusion 2,867 - 0.19%, Vasculitis 970 - 0.07%
KEYTRUDA can cause severe or fatal cases for some of these adverse reactions: Nervous System: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy;
COVID-19 “vaccines”: Meningitis 373 - 0.03%, Meningitis aseptic 203 - 0.01%, Meningitis bacterial 38 - 0%, Encephalitis 654 - 0.04%, Encephalitis allergic 3 - 0%, Encephalitis autoimmune 126 - 0.01%, Encephalitis brain stem 20 - 0%, Myelitis 551 - 0.04%, Myelitis transverse 564 - 0.04%, Demyelinating polyneuropathy 130 - 0.01%, Demyelination 327 - 0.02%, Myasthenia gravis 534 - 0.04%, Myasthenia gravis crisis 37 - 0%, Myasthenic syndrome 30 - 0%, Guillain-Barre syndrome 3,178 - 0.21%, Neuropathy peripheral 3,546 - 0.24%, Autoimmune neuropathy 29 - 0%
KEYTRUDA can cause severe or fatal cases for some of these adverse reactions: Ocular: Uveitis, iritis and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur.
COVID-19 “vaccines”: Uveitis 664 - 0.04%, Uveitis-glaucoma-hyphaema syndrome 2 - 0%, Iritis 184 - 0.01%, Retinal detachment 410 - 0.03%, Retinal artery occlusion 379 - 0.03%, Retinal haemorrhage 239 - 0.02%, Blindness 2,310 - 0.16%, Blindness congenital 1 - 0%, Blindness cortical 8 - 0%, Blindness transient 540 - 0.04%, Blindness traumatic 1 - 0%, Blindness unilateral - 861 - 0.06%
KEYTRUDA can cause severe or fatal cases for some of these adverse reactions: Gastrointestinal: Pancreatitis, to include increases in serum amylase and lipase levels, gastritis, duodenitis;
COVID-19 “vaccines”: Pancreatitis 611 - 0.04%, Pancreatitis acute 589 - 0.04%, Pancreatitis chronic 28 - 0%, Pancreatitis haemorrhagic 1 - 0%, Pancreatitis necrotising 44 - 0%, Gastritis 700 - 0.05%, Duodenitis 43 - 0%
KEYTRUDA can cause severe or fatal cases for some of these adverse reactions: Musculoskeletal and Connective Tissue: Myositis/polymyositis, rhabdomyolysis (and associated sequelae, including renal failure), arthritis (1.5%), polymyalgia rheumatica;
COVID-19 “vaccines”: Myositis 676 - 0.05%, Polymyositis 72 - 0%, Rhabdomyolysis 697 - 0.05%, Renal failure 1,321 - 0.09%, Arthralgia 81,922 - 5.5%, Arthritis 3,783 - 0.25%, Arthritis allergic 3 - 0%, Arthritis bacterial 61 - 0%, Arthritis enteropathic 6 - 0%, Arthritis gonococcal 1 - 0%, Arthritis infective 41 - 0%, Arthritis reactive 507 - 0.03%, Polymyalgia rheumatica - 1,769 - 0.12%
KEYTRUDA can cause severe or fatal cases for some of these adverse reactions: Endocrine: Hypoparathyroidism;
COVID-19 “vaccines”: Hypoparathyroidism 8 - 0%
KEYTRUDA can cause severe or fatal cases for some of these adverse reactions: Hematologic/Immune: Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection.
COVID-19 “vaccines”: Haemolytic anaemia 183 - 0.01%, Aplastic anaemia 116 - 0.01%, Haemophagocytic lymphohistiocytosis 156 - 0.01%, Systemic inflammatory response syndrome 394 - 0.03%, Histiocytic necrotising lymphadenitis 22 - 0%, Sarcoidosis 339 - 0.02%, Immune thrombocytopenia 1,548 - 0.1%, organ transplant rejection: https://www.nature.com/articles/s41541-022-00445-5, https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.32409, https://www.cureus.com/articles/80149-a-case-of-hepatotoxicity-after-receiving-a-covid-19-vaccine
Based on its mechanism of action, KEYTRUDA can cause fetal harm when administered to a pregnant woman.
COVID-19 “vaccines”: Abortion spontaneous 3,515 - 0.24%, Stillbirth 153 - 0.01%,
KEYTRUDA can result in increased mortality.
COVID-19 “vaccines”: Death 17,517 - 1.18%
The people behind these injections knew their ingredients well. They knew the list of side effects but did not disclose them because they were "legally protected."
FDA was provided with this short list: https://www.fda.gov/media/143557/download
“FDA Safety Surveillance of COVID-19 Vaccines: DRAFT Working list of possible adverse event outcomes ***Subject to change***, page 16 - BUT IT WASN'T PUBLIC








Lord help us, because no one else will.
"Do No Harm" has been trashed by Big Pharma manipulated medical research that inflates the positive effects and hides the great harm that is suffered by patients. Pfizer's reports of over 1000 negative health effects from the mRNA injections which included death was ignored! Dr. Breggins words: "We are the Prey" ring true! There is a trail of medical deaths caused by FDA approved drugs over the last 50 years that is a mile wide and no action has been taken by our DC leadership to stop the carnage. FDA has allowed about 500 RX drugs that cause death to remain on the market and have just put a BLACK BOX Warning on them in the PDR. Hundreds of medical professionals have spoken out on the need for independent oversight of the drug approval process and their words have fallen on deaf ears.