Robert Malone, the $5 billion contract
AND CATIONIC LIPIDS
DO WE REALLY BELIEVE IN “OPERATION WARP SPEED”?
https://www.cell.com/iscience/fulltext/S2589-0042(21)01450-4?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2589004221014504%3Fshowall%3Dtrue The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory: iScience (cell.com)
With the experiments presented earlier, we observed a high mortality rate among the mice inoculated with LNPs.
Intradermal and intramuscular inoculation with LNPs induces robust inflammation…
https://www.forbes.com/sites/williamhaseltine/2020/09/23/covid-19-vaccine-protocols-reveal-that-trials-are-designed-to-succeed/?sh=d1dd55452479 Covid-19 Vaccine Protocols Reveal That Trials Are Designed To Succeed | Sep 23, 2020
This unusually transparent action during a major drug trial deserves praise, close inspection of the protocols raises surprising concerns.
These trials seem designed to prove their vaccines work, even if the measured effects are minimal.
What would a normal vaccine trial look like?
Prevention of infection must be a critical endpoint.
Prevention of infection is not a criterion for success for any of these vaccines.
In fact, their endpoints all require confirmed infections and all those they will include in the analysis for success,
the only difference being the severity of symptoms between the vaccinated and unvaccinated.
Three of the vaccine protocols—Moderna, Pfizer, and AstraZeneca—do not require that their vaccine prevent serious disease only that they prevent moderate symptoms which may be as mild as cough, or headache. (!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!)
None list mortality as a critical endpoint.
Vaccine efficacy is typically proved by large clinical trials over several years. The pharmaceutical companies intend to do trials ranging from thirty thousand to sixty thousand participants.
The first surprise found upon a closer reading of the protocols reveals that each study intends to complete interim and primary analyses that
at most include 164 participants. (!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!)
For Moderna, the initial interim analysis will be based on the results of infection of only 53 people. The judgment reached in interim analysis is dependent upon the difference in the number of people with symptoms, which may be mild, in the vaccinated group versus the unvaccinated group.
For Johnson & Johnson, their interim analysis includes 77 vaccine recipients, with a success margin of 18 or less developing symptoms compared to 59 in the control group. For AstraZeneca, their interim analysis includes 50 vaccine recipients, with a success margin of 12 or less developing symptoms compared to 19 in the 25 person control group. Pfizer is even smaller in its success requirements. Their initial group includes 32 vaccine recipients, with a success margin of 7 or less developing symptoms compared to 25 in the control group.
The second surprise from these protocols is how mild the requirements for contracted Covid-19 symptoms are. A careful reading reveals that the minimum qualification for a case of Covid-19 is a positive PCR test and one or two mild symptoms. These include headache, fever, cough, or mild nausea.
This is far from adequate. These vaccine trials are testing to prevent common cold symptoms. (!!!!!!!!!!!!!!!!!!!!!!!!!!!!!)
It appears that all the pharmaceutical companies assume that the vaccine will never prevent infection. Their criteria for approval is the difference in symptoms between an infected control group and an infected vaccine group.
[https://www.youtube.com/watch?v=mnxlxzxoZx0 Pfizer did not know whether Covid vaccine stopped transmission before rollout - YouTube]
None list the prevention of death and hospitalization as a critically important barrier.
There must be an indication that the authorized vaccines are reducing infection, hospitalization, and death, or else they will not be able to stop this pandemic.
These protocols do not emphasize the most important ramifications of Covid-19 that people are most interested in preventing: overall infection, hospitalization, and death. It boggles the mind and defies common sense that the National Institute of Health, the Center for Disease Control, the National Institute of Allergy and Infectious Disease, and the rest would consider the approval of a vaccine that would be distributed to hundreds of millions on such slender threads of success.
It appears that these trials are intended to pass the lowest possible barrier of success. (!!!)
“Dr. Yager said that thanks to advances in genomic sequencing, researchers successfully uncovered the viral sequence of SARS-CoV-2 in January 2020 — roughly 10 days after the first reported pneumonia cases in Wuhan, China.”
However, FOIs reveal that health/science institutions around the world (213 and counting!) have no record of SARS-COV-2 isolation/purification, anywhere, ever https://www.fluoridefreepeel.ca/fois-reveal-that-health-science-institutions-around-the-world-have-no-record-of-sars-cov-2-isolation-purification/
https://www.businessinsider.com/moderna-designed-coronavirus-vaccine-in-2-days-2020-11?IR=T
On January 11, researchers from China published the genetic sequence of the coronavirus.
Two days later, Moderna's team and NIH scientists had finalized the targeted genetic sequence they would use in the vaccine.
By February 24, Moderna had shipped its first vaccine batches to NIH scientists in Bethesda, Maryland.
“researchers from China published the genetic sequence of the coronavirus. “ - WHY DIDN'T THEY TAKE A SAMPLE FROM THE PATIENT AND SEQUENCE IT?
BUT THEY WERE READY TO GO
https://inside.battelle.org/blog-details/bringing-down-barriers-to-medical-countermeasure-development Bringing Down Barriers to Medical Countermeasure Development (battelle.org)
In 2013, the DoD contracted with Nanotherapeutics Inc. to create the organization as a shared resource for companies working on the medical countermeasures the DoD needs. The ADMc has state-of-the-art manufacturing facilities for vaccines and biologics to help smaller companies scale up from R&D to manufacturing.
The ADMc also is prepared to support the production of
countermeasures for pandemic diseases.
GIVEN THIS:
”On January 11, researchers from China published the genetic sequence of the coronavirus.
Two days later, Moderna's team and NIH scientists had finalized the targeted genetic sequence they would use in the vaccine.”
WHAT DELIVERY PLATFORM WAS USED FOR THESE INJECTIONS?
https://cen.acs.org/pharmaceuticals/drug-delivery/Without-lipid-shells-mRNA-vaccines/99/i8 Without these lipid shells, there would be no mRNA vaccines for COVID-19 (acs.org)
Fragile mRNA molecules used in COVID-19 vaccines can’t get into cells on their own. They owe their success to lipid nanoparticles that took decades to refine.
On top of that, mRNA strands are large and negatively charged and can’t simply waltz across the protective lipid membranes of cells. Many scientists thought the technology would never work.
Luckily, scientists found a solution. To protect the fragile molecule as it sneaks into cells, they turned to a delivery technology with origins older than the idea of mRNA therapy itself: tiny balls of fat called lipid nanoparticles, or LNPs.
LNPs used in the COVID-19 vaccines contain just four ingredients: ionizable lipids whose positive charges bind to the negatively charged backbone of mRNA, pegylated lipids that help stabilize the particle, and phospholipids and cholesterol molecules that contribute to the particle’s structure.
It is a tremendous vindication for everyone working in controlled drug delivery.
Robert Langer, chemical engineer, Massachusetts Institute of Technology
Over more than 3 decades, promising lipids studied in the lab often failed to live up to their potential when tested in animals or humans. Positively charged lipids are inherently toxic, and companies struggled for years before landing on formulations that were safe and effective. When injected intravenously, the particles invariably accumulated in the liver, and delivery to other organs is still an obstacle. Reliably manufacturing consistent LNPs was another challenge, and producing the raw materials needed to make the particles is a limiting factor in the production of COVID-19 vaccines today.
“LNPs will be going into millions of arms over the course of this year,” says University of British Columbia nanoparticle scientist Pieter Cullis.
Modern LNPs can be traced back to work on simpler systems called liposomes, hollow lipid spheres often made of just two or three kinds of lipids. In the early 1980s, Cullis found that cancer drugs could diffuse into these liposomes and get trapped in the hollow core. When injected into animals with cancer, the liposomes would slip through the leaky vasculature of tumors, enter cells, and unleash a drug.
“There are no cationic lipids in nature,” Cullis says. “And we knew we couldn’t use permanently positively charged lipids because they are so damn toxic.” Those lipids would rip cell membranes apart, he adds.
Protiva found that one of its experimental LNP therapies caused a more severe immune reaction in humans than it had in the lab, and the company pinned pegylated lipids as a major factor.
“There was this time when LNPs went through the dark ages,” says Thomas Barnes, CEO of the mRNA company Orna Therapeutics. “I think there is going to be a bit of a renaissance in these ionizable lipids and that the world is going to get excited about LNPs again.”
IN SHORT, IT IS ABOUT INJECTING THOSE LNPS THAT HAVE BEEN IN USE FOR A LONG TIME (IN CANCER AND GENE THERAPIES), INTO THE ARM OF EVERY HUMAN BEING.
ITS TOXICITY IS WELL KNOWN
https://www.tandfonline.com/doi/full/10.2147/IJN.S73017 Full article: Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: lipid nanoparticles versus polymeric nanoparticles (tandfonline.com)
Conclusion
Cationic additives in nanoparticles could trigger cell death and toxic responses. Inflammatory mediators of PMNs including elastase and intracellular Ca2+ were increased by cationic nanoparticles. These nanosystems also induced O2•− generation. The mechanisms of cytotoxicity or inflammation caused by cationic nanoparticles can be proposed based on experimental data in the present study. The lipid rafts in the plasma membrane are the targets of nanoparticles. The particles interact with the cell membrane, triggering membrane disruption and leakage. Intracellular Ca2+ is increased due to this disruption, leading to O2•− generation. Both Ca2+ and O2•− elevation can induce degranulation of PMNs. The cell death occurs because of the production of oxidative stress and excitation of inflammation.
https://www.news-medical.net/news/20210315/Research-looks-at-inflammatory-nature-of-lipid-nanoparticle-component-in-mRNA-vaccines.aspx Research looks at inflammatory nature of lipid nanoparticle component in mRNA vaccines (news-medical.net)
In a recent bioRxiv* preprint research paper, Botond Z. Igyártó and colleagues from Thomas Jefferson University demonstrated the inflammatory nature of the lipid nanoparticles (LNPs). Upon intradermal administration in mice, they observed
massive neutrophil infiltration, activation of diverse inflammatory pathways, and production of various inflammatory cytokines and chemokines.
They observed similar observations when the LNPs were delivered intranasally.
https://www.biorxiv.org/content/10.1101/2021.03.04.430128v1The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory | bioRxiv
Here we present evidence that LNPs used in many preclinical studies are highly inflammatory in mice. Intradermal injection of these LNPs led to rapid and robust inflammatory responses, characterized by massive neutrophil infiltration, activation of diverse inflammatory pathways, and production of various inflammatory cytokines and chemokines. The same dose of LNP delivered intranasally led to similar inflammatory responses in the lung
and resulted in a high mortality rate. (!!!!!!!!!!!!!!!!!!!!!!!!!!!!!)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC297778/ Cationic liposome-mediated RNA transfection - PubMed (nih.gov) R W Malone 1, P L Felgner, I M Verma
We have developed an efficient and reproducible method for RNA transfection, using a synthetic cationic lipid…
https://pubmed.ncbi.nlm.nih.gov/8867864/ Structural and functional analysis of cationic transfection lipids: the hydrophobic domain - PubMed (nih.gov) R P Balasubramaniam 1, M J Bennett, A M Aberle, J G Malone, M H Nantz, R W Malone

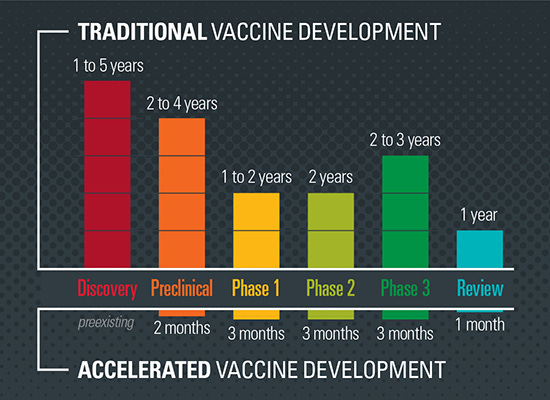







Let's face it. This is what Dr. Malone can't discuss for many reasons. This was a DOD/CIA/NIH biowarfare exercise which benefited Big Pharma, the eugenists and the Globalists.
This is the most important interview that RJK Jr. has ever done and should cause everyone who cares about the future of humanity to be outraged.
https://live.childrenshealthdefense.org/chd-tv/shows/the-defender-show/militarized-healthcare/?utm_source=email&utm_medium=salsa&utm_campaign=CHDTV&eType=EmailBlastContent&eId=90797074-5f47-46f0-9228-b363a45276be
Outraged and all,
I love all the enthusiasm of everyone sharing very important solid research/evidence proving the treasonous BIOWEAPONS event…necessary for serious accountability of the maniacal global psychopaths!
My focus…HOW do you stop ALL the dangerous Treason after 3 LONG years of so many suffering, including this young lady pilot?
Nothing good can happen in America until there is UNITY of the right people to do things that matter to restore our Constitutional Republic.
Keep up with serious writer Lex Greene @News with VIEWS who writes for those who value Freedom and Liberty … UNITY 101 - E PLIRIBUS by Lex Greene
https://newswithviews.com/unity-101-e-pluribus-unum
Respectfully,
A Fierce Mother/grandmother Lion (of 6 and counting!
P.S. Divided…our house is falling!