MICROCHIPING PEOPLE IS THE GOAL
https://anamihalceamdphd.substack.com/cp/137384529
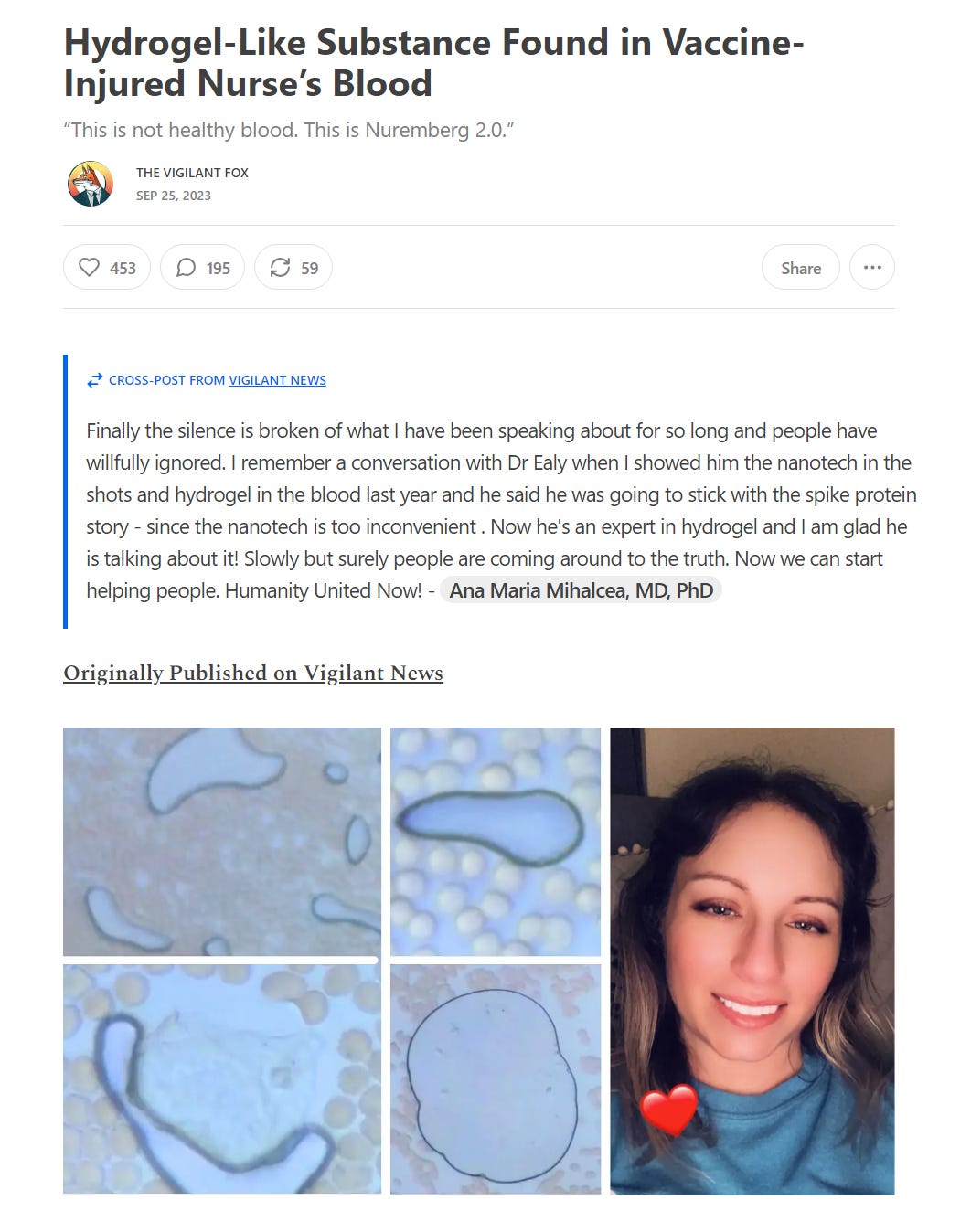
https://vigilantnews.com/post/hydrogel-like-substance-found-in-vaccine-injured-nurses-blood
MICROCHIPING PEOPLE IS THE GOAL
THAT'S WHY THERE ARE HYDROGELS IN THE BLOOD AND WHY PEOPLE HAVE SERIOUS ADVERSE EFFECTS AND DIE, BECAUSE THESE TECHNOLOGIES ARE TOXIC.
http://web.archive.org/web/20130523013419/http://proteusdigitalhealth.com/company/partners
First published on October 9, 2009 / 1:56 PM
© 2009 CBS Interactive Inc. All Rights Reserved.
The news that Novartis wants a deal with Proteus Biomedical to produce a microchip called "Raisin" that will text your mobile phone when it's time to take another pill, and VeriChip's efforts to link microchip implants to online health records, has caused two separate controversies that seem bound to collide: some Christians believe the devices are eerily similar to the "mark of the beast" as described in the book of Revelation; while "singularity" buffs -- those who look forward to the merger of humans and intelligent technology -- regard it as a bold step forward in improving health.
On the other hand, the singularity buffs see nothing but good news. There's an obvious advantage on the club scene,
as VeriChip could replace both photo ID and cash:
Beautiful club-goers have a problem: If you're going to wear a halter top and micro-skirt, there's not much of anywhere to put a wallet. And who wants to carry a purse when you're there to dance?
Luckily, a company called VeriChip this year unveiled a solution based on radio-frequency identification (RFID) technology.
Raisin, or any system that helps us discipline our health habits is bound to help us live longer and happier. That's the promise of Body 2.0 and I hope that the partnership between Proteus and Novartis means that promise is gaining ground in the global marketplace.
And finally:Proteus CEOAndrew Thompsonbelieves his company's market opportunity could be $100 billion. This is delusional. The serious debate here is over privacy and tracking concerns, and whether anyone might be required to have an implant. The vast majority of patients and consumers simply won't want one.
By Jim Edwards | Oct 5, 2009
Novartis and Proteus Biomedical are not the only companies hoping to implant microchips into patients so that their pill-popping habits can be monitored. VeriChip of Delray Beach, Fl., has an even bolder idea: an implanted chip that links to an online database containing all your medical records, credit history and your social security ID.
The VeriMed Health Link homepage describes the chip:
… a tiny, passive microchip (the nation’s first and only microchip cleared for patient identification by the U.S. Food & Drug Administration) and a secure, private online database that links you to your personal health record. Your Health Link is always with you and cannot be lost or stolen.
It’s a future in which your doctor tags you like a dog with a microchip that allows anyone with the right privileges to look at your medical records, credit history, social security number (see slide 6), and anything else that stems from that.
Novartis implanted computer chips into the shoulders of 20 patients taking the blood pressure drug Diovan; the chips sent text messages to their cellphones when it was time to take the next pill. The experiment was designed to improve “compliance.” (Lousy compliance is the phenomenon of patients receiving prescriptions but not filling or taking them — thus costing Big Pharma sales.)
The development will be sure to horrify conspiracy theorists, civil libertarians, privacy activists, paranoid schizophrenics and anyone else who does not want a computer chip monitored by a multinational drug company inserted into their body.
Novartis’ efforts are doubly creepy because it actually involves two internal chips. The first chip is inside the pill being swallowed. It sends a signal to the chip in your shoulder. If you fail to take your next pill, the shoulder chip nags you on your mobile. Note that after the text arrives on your phone, the message then goes “onto the internet for caregivers to review and analyze.”
Creepiest of all? It works, according to the FT:
Joe Jimenez, head of pharmaceuticals at Novartis, said tests using the system – which broadcasts from the “chip in the pill” to a receiver on the shoulder – on 20 patients using Diovan, a drug to lower blood pressure, had boosted “compliance” with prescriptions from 30 per cent to 80 per cent after six months.
And finally: “Pfizer’s Health Solutions division has developed a system to telephone patients to encourage them to take medicine,” the FT notes.
Posted 5/12/2004 12:40 AM Updated 5/12/2004 3:07 AM
Once implanted, you become your own credit card.
Need to pay for a drink? Wave your implant near a reader, and you're done. VeriChip has dreams of going global with its "human implantable ID technology" — once implanted, you could wave a body part to pay for a burger at Wendy's, a beer at a baseball game, or whatever.
There are a few kinks to be worked out, like the fact that you can't turn the chip off. Privacy groups are going to dog-pile on that one.
Another company is taking the idea of implanted radio-enabled chips to a different level. Cyberkinetics of Foxborough, Mass., calls itself "a leader in the rapidly emerging field of brain computer interfaces." The company makes BrainGate — which, despite the 1974 analogies here, is not a reference to a scandal involving someone's brain.
When implanted in a person's brain, the device can allow that person to control a computer just by thinking. It is essentially a mouse moved by brain waves. Last month, the company got federal approval to implant the chips in five paralyzed people as a test.
While the first uses of BrainGate would be to help the paralyzed, certainly such devices could eventually be implanted in healthy people. The military has visions of pilots flying planes by thought. Imagine what the porn industry — always on tech's cutting edge — could do with hands-free computing.
Another recent development suggests that people might someday be able to see in the dark. Earlier this year, Raytheon announced its Thermal-Eye 2600AS technology. This allows thermal-imaging cameras — the kind that lets people see at night or through smoke — to be small enough to be built into a firefighter's helmet. Instead of a bulky camera, thermal imaging can become almost a part of a firefighter.
The company says the technology can keep getting smaller and better.
Progress in bionics isn't just about putting electronics into humans. In some cases, it's about putting humanness into electronics.
There will be a lot more news about the merging of machines and humans. University labs are doing research. Companies are being started.
Tuesday - September 22nd, 2009 - 05:45pm EST by Brian Dolan | intelligent medicine | Novartis | pharmaceuticals | Proteus Biomedical | wireless remote monitoring |
Pharmaceutical giant Novartis has tapped intelligent medicine start-up Proteus Biomedical for a small 20 patient study to track patients’ compliance with their blood pressure drug regimen.
The patients are taking blood pressure drug Diovan and the study organizers track their compliance via Proteus’ “chip in the pill” technology, which reports to a receiver sensor on the patient’s shoulder when the medication has been ingested. The study has improved compliance from 30 percent to 80 percent after six months, according to Novartis.
“This industry is starting to explode,” Joe Jimenez, head of pharmaceuticals at Novartis, told the Financial Times. Jimenez is toying with the idea of hiring a “compliance tsar” to oversee the company’s partnerships and initiatives in this area. Jimenez told the newspaper that challenges to widespread adoption of Proteus’ technology for Novartis would include the obvious regulatory concerns, but, interestingly, Jimenez stressed that Novartis would also have to “negotiate an exclusive contract with Proteus in order to expand the approach,” according to the report.
An exclusive deal with Novartis? That would be a huge deal for Proteus Biomedical, but during a panel session at CTIA last year that MobiHealthNews helped organized, Proteus CEO Andrew Thomspon pegged his company’s market opportunity at $100 billion.
Regardless, news of Novartis’ trial with Proteus Biomedical is a huge boon for intelligent medicine, personalized medicine, compliance solutions and advanced medical sensors.
“We’ll do all of this for the same price as the drugs you buy now,” Thompson explained during an interview earlier this year. “For one daily price of your medicine you get the drug, the monitoring, the applications and tools, the incentives and the connectivity.”
The system is being developed by Novartis, the pharmaceutical company, in partnership with a technology company called Proteus Biomedical.
https://www.gatesfoundation.org/about/committed-grants/2012/04/opp1054487
https://www.gatesfoundation.org/about/committed-grants/2022/08/inv048993
The Bill and Melinda Gates Foundation funded the team's research, which was published in the journal Science Translational Medicine on Wednesday.
According to a Scientific American story, the project came about following a direct request from Microsoft founder Bill Gates himself, who has been personally involved in efforts to eradicate polio and measles through vaccinations.
The invisible "tattoo" accompanying the vaccine is a pattern made up of minuscule quantum dots - tiny semiconducting crystals that reflect light - that glows under infrared light. The pattern - and vaccine - gets delivered into the skin using hi-tech dissolvable microneedles made of a mixture of polymers and sugar.
http://web.archive.org/web/20191220214301/https://stm.sciencemag.org/content/11/523/eaay7162
MOSIP is an open source and open standards-based foundational digital ID platform. It is modular and API-based. This means any particular component can be replaced, with no vendor lock-in issues. It is funded by the Omidyar Network and
the Bill & Melinda Gates Foundation
as well as India’s Tata Trust. It is governed by an Executive Committee and Technology Committee and has an International Advisory Group with members such as UNHCR, ID4D, ID2020 and ID4Africa.
Overall, the report assesses MOSIP as a sure step towards establishing an identity system based on privacy and which can be continually updated. Though there is no guarantee that a country would enforce these principles of engagement.
https://www.gatesnotes.com/Heroes-in-the-Field-Nandan-Nilekani
MARCH 3, 2020
The Defense Department is helping to fund a new study to determine whether an under-the-skin biosensor can help trackers keep up — by detecting flu-like infections even before their symptoms begin to show. Its maker, Profusa, says the sensor is on track to try for FDA approval by early next year.
The sensor has two parts. One is a 3mm string of hydrogel, a material whose network of polymer chains is used in some contact lenses and other implants. Inserted under the skin with a syringe, the string includes a specially engineered molecule that sends a fluorescent signal outside of the body when the body begins to fight an infection. The other part is an electronic component attached to the skin. It sends light through the skin, detects the fluorescent signal and generates another signal that the wearer can send to a doctor, website, etc.
https://news.un.org/en/story/2019/06/1040131
The 20-strong High Level Panel on Digital Cooperation is co-chaired by Jack Ma and Melinda Gates, with members drawn from a diverse group of independent experts, including US internet pioneer Vint Cerf, and South Korea-based digital marketing mastermind Sophie Eom. The Panel was created to fulfil the UN chief’s wish to include input from industry and the private sector, as well as governments, academia, civil society and inter-governmental organizations, in tackling the challenges of the digital age.
This work was funded by the Bill and Melinda Gates Foundation, the Government of Estonia, Fondation Botnar, the State of Kuwait, and the Rockefeller Foundation. The views of the funding bodies have not influenced the content of this document.
SHALL: SHALL is used to describe technical features and functions that are mandatory for this specification.1
A digital vaccination certificate that documents a person’s current vaccination status to protect against COVID-19 (?????????????????) can then be used for continuity of care or as proof of vaccination for purposes other than health care. (!!!!!!!!!!!!!!!!!!!!!!)
As we know these injections NEVER PROTECTED AGAINST so-called COVID.
https://twitter.com/Rob_Roos/status/1580194898791354371 “Pfizer executive just told me that at the time of introduction, the vaccine was never tested to stop virus transmission.
"This removes the entire legal basis of the Covid passport - the Covid passport that has led to massive institutional discrimination, as people have lost access to essential parts of society," Roos added. "I find it shocking, even criminal.”
Roos shared his exchange with Janine Small.
"I will speak in English so that there are no misunderstandings," Roos said. "Was Pfizer's Covid vaccine tested to stop virus transmission before it entered the market? If not, please say so clearly. If yes, are you willing to share data with this committee? And I really want a straight yes or no answer, and I'm looking forward to it."
"As for the question, did we know about stopping immunization before it was essentially on the market?" Pfizer executive J. Small admitted: "No... We really had to act at the speed of science to really understand what was happening in the market."
In fact, “Covid” is reported as adverse effect in VAERS:
https://medalerts.org/vaersdb/findfield.php?TABLE=ON&GROUP1=SYM&EVENTS=ON&VAX=COVID19
COVID-19 207,166 13.3%
But WHO CARES??? It is JUST about injecting people, not about any “Covid”.
Sep 23, 2020,01:11pm EDT
These trials seem designed to prove their vaccines work, even if the measured effects are minimal.
What would a normal vaccine trial look like?
Prevention of infection must be a critical endpoint.
Prevention of infection is not a criterion for success for any of these vaccines.
The greatest fear people have is dying from this disease.
None list mortality as a critical endpoint.
The first surprise found upon a closer reading of the protocols reveals that each study intends to complete interim and primary analyses that at most include 164 participants.
A careful reading reveals that the minimum qualification for a case of Covid-19 is a positive PCR test and one or two mild symptoms. These include headache, fever, cough, or mild nausea. This is far from adequate. These vaccine trials are testing to prevent common cold symptoms.
https://www.ledgerinsights.com/id2020-resignation-blockchain-covid-19-immunity-passports/
Elizabeth M. Renieris, a Harvard lawyer on the ID2020 technical advisory committee, has resigned from the ID2020 Alliance.
The blog post written ten days ago did not refer to ID2020 but does mention the COVID Credentials Initiative, which it says “is led by for-profit companies eager for a use case for their as-yet unadopted technologies. Notably, participants do not include any public health experts.”
So the big question is whether there should be immunity passports at all. And that’s the core of Renieris’ argument.
The legal hurdle
On the legal front, the blog post cites how in China, individuals have to demonstrate a green QR code of health status to access public transport and work. To wave fundamental rights of freedom of assembly, movement, work, and more, it’s argued there is a need to satisfy a three part test. The interference must be in accordance with the law, necessary to achieve a certain aim and proportionate to the aim pursued. And to suspend civil liberties, it should be evidence-based.
“At this time, we know of no specific or general legal frameworks which would provide individuals with sufficient clarity and precision as to how any data processed in connection with such blockchain-enabled immunity credentials would be governed or processed, or that could provide individuals with sufficient safeguards or protections in respect of their use,” the blog post states.
The Proteus Digital Health™
“Data can be viewed and shared in a multitude of ways. Some of the data dissemination methods that are currently being implemented include web-based access using a computer or a smartphone, email notification, and text messaging.”
http://web.archive.org/web/20130224014543/http://proteusdigitalhealth.com/technology/
Proteus is privately held and funded by Carlyle, Essex Woodlands, Kaiser Permanente®, Medtronic®, Novartis®, Otsuka®, Oracle®, ON Semiconductor® and other investors.
LOS ANGELES – The University of Southern California (USC) Center for Body Computing (CBC), the digital health innovation accelerator for the Keck Medicine of USC medical enterprise, today announced its eight foundational partners for its Virtual Care Clinic (VCC). The disruptive digital health care model does not require patient or care providers to be present in the same place for seamless, integrated solutions designed to provide on-demand access to care. The VCC extends Keck Medicine of USC experts to anyone with a smartphone by harnessing cutting-edge technologies and creative solutions developed at the renown USC Institute of Creative Technologies (ICT) in Playa Vista, the heart of Los Angeles’ digital zone known as Silicon Beach.
In addition to its collaboration with ICT, the USC CBC invited the following best-in-class foundational partners to establish its VCC ecosystem: Doctor Evidence, IMS Health, Karten Design, Medable, Planet Grande, Proteus Digital Health and VSP Global. Using mobile apps, “virtual doctors,” data collection and analysis systems, world-class diagnostic and wearable sensors coupled with experiential design and engaging, expert patient health information, the VCC delivers wireless, on-demand access to Keck Medicine of USC experts while doctors go beyond telemedicine models for remote management and care of patients regardless of location.
“Our Virtual Care Clinic is not only the democratization of health care allowing anyone access to our medical experts without leaving their home, but it also capitalizes on the promise that digital health is supposed to offer,” said Leslie Saxon, MD.
Innovative patient care models such as our VCC will create operational efficiencies and cost-savings allowing us to refocus resources back into more innovation and constantly improve the patient experience. This is redefining medical care.”
The digital health sector – which encompasses mobile health, remote monitoring through smartphone-enabled devices or apps, sensors, and other wireless health solutions – has seen exponential growth over the last four years with 2015 venture funding in the space totaling $4.5 billion according to Rock Health. Two-thirds of all Americans, 200 million people, own a smartphone according to Pew Research and a report by MobileFuture stated use of mobile devices as health tools and remote patient monitoring could save the U.S an estimated $36 billion in health care costs by 2018.
MEET OUR EXACUTIVE TEAM - REVOLVING DOORS BETWEEN BIG PHARMA, BANKING INDUSTRY, GOVERNMENT OFFICES, ACADEMIA AND WEF
Andrew Thompson serves on the World Economic Forum Technology Pioneers selection committee and he is also a Co-Founder and Board member of Summit Schools, a leading Charter High School organization with a unique digital platform. Andrew is active in digital humanities innovation with Stanford University and Cambridge University and a Co-Founder of Parker Library Online – the leading destination for digital medieval studies. He also serves on the California Governor’s Health IT Security Advisory Board.
George Savage serves on the board of the California Life Sciences Association, the Boston University College of Engineering advisory council, and in 2016 was elected a Fellow of the American Institute for Medical and Biological Engineering.
Molly O’Neill is a member of the board of World Care International Inc. Molly has also held leadership roles at Duke Medicine, Partners Healthcare, Inova Health System, Ascension Health Care Network, a joint venture between Ascension Health Alliance and Oak Hill Capital Partners.
Neela Paykel advised on a broad range of managed care, healthcare law, privacy, antitrust and FDA promotional issues.
Meet our Board of Directors
Jonathan Symonds, CBE Chairman
Jon Symonds, CBE, is Chairman of HSBC Bank Plc, Chairman of the Supervisory Board of Innocoll AG and Non-Executive Director of Genomics England. He serves on the boards of Tala Energy, Feliz Pharmaceuticals, Aetion Inc., and Mesoblast.
Formerly, Symonds was Chief Financial Officer of Novartis, Partner and Managing Director of Goldman Sachs and Chief Financial Officer of AstraZeneca Plc.
Shumeet Banerji, Ph.D. Board Member
Shumeet Banerji is founder and partner of Condorcet, LP an investment and advisory firm. He serves on the Board of HP, Inc., Reliance Industries Limited, Proteus Digital Health, Inc., Felix Pharmaceuticals Private Limited, and Tala Energy Private Limited.
Regina Benjamin, MD, MBA Board Member
Dr. Regina Benjamin is the Founder and CEO of BayouClinic in Bayou La Batre, Alabama, and the NOLA.com/Times Picayune Endowed Chair of Public Health Sciences at Xavier University of Louisiana. She was the 18th United States Surgeon General (2009 – 2013).
Dr. Benjamin serves on the boards of Kaiser Foundation Health Plan and Hospitals, the largest U.S. health managed care organization, and of Ascension, the world’s largest faith-based health system. She also serves on the boards of Diplomat, ConvaTec, CPSI, Next Level Health, and 98point6.
Robert Epstein, M.D., M.S. Board Member
Dr. Robert Epstein is the cofounder and CEO of Epstein Health, LLC, a life science strategic consulting firm serving clients in the United States and Europe.
Dr. Epstein is the former president of the International Society of Pharmacoeconomics and Outcomes Research, and currently serves on the board of directors of Illumina, Fate Therapeutics, Veracyte, and Mindstrong Health.
Frank Fischer Board Member
Mr. Fischer continues to serve on the boards of several privately held medical device companies, in addition to serving on the board of trustees of Epilepsy Foundation of America, Rensselaer Polytechnic Institute, and Babson College.
Alan J. Levy, Ph.D. Board Member
Dr. Alan Levy is a venture partner with Frazier Healthcare Partners and has over 30 years of senior management experience in the medical device industry.
Dr. Levy serves on the board of Intuitive Surgical, a publicly held company, in addition to Chrono Therapeutics, AbyRx, Signum Surgical, and the Center for Infectious Disease Research.
Myrtle Potter Board Member
Myrtle Potter is founder and CEO of Myrtle Potter and Company. A best-selling author, Ms. Potter has dedicated three decades to serving the healthcare needs of millions of consumers.
She serves on the board of Rite Aid Corp., Liberty Mutual Insurance Group, and Insmed, Inc., and is on the University of Chicago Board of Trustees. Ms. Potter was president and chief operating officer of Genentech, president at Bristol-Myers Squibb and a vice president at Merck.
Ryan Schwarz Board Member
Mr. Schwarz currently serves on the boards of Lucent Health Solutions, Argos Health, and ORN Healthcare.
Charles Songhurst Board Member
Mr. Songhurst serves on the boards of Rigetti, SafeGraph, and CodeFights. Previously, he ran corporate strategy for Microsoft and focused on partnering and M&A.
Joseph R. Swedish Board Member
Joseph R. Swedish is Executive Chairman of Anthem Inc., a Fortune 50 company, and the nation’s leading health benefits provider.
Formerly, Swedish served as CEO for several major integrated healthcare delivery systems, including Trinity Health, Colorado’s Centura Health, and Hospital Corporation of America.
Swedish serves on numerous corporate and industry association boards, and joined the Proteus Board of Directors in February 2018.
Partners
Proteus develops solutions in partnership with leading companies in a variety of industries. Please contact us if your organization is interested in partnering to become a digital health leader.
Current partners include:
Btw.: Dr. RW Malone co-founded and helped to secure $2.3 million in V.C. funding, including monies from the Novartis Venture Fund (Novartis Venture Fund: Founded in 1996, Novartis Venture Fund is a corporate venture capital investment arm of Novartis headquartered in Basel, Switzerland. The firm has a regional office in Cambridge, Massachusetts. The firm seeks to invest in the life sciences, oncology, biotechnology, and digital health sectors. https://static1.squarespace.com/static/617d909092cd2314e37ebe67/t/618a94e99a3e6950214f54ed/1636472043910/RWM+CV+6+Aug+2021+for+website.doc
Our system received European regulatory approval (CE Mark) in August 2010. The patch received U.S. FDA market clearance as a medical device in April 2010. The ingestible sensor received U.S. FDA market clearance as a medical device for co-ingested applications in July 2012. For more information on the technology, please reference this publication.
Kit Yee Au-Yeung's research while affiliated with Proteus Digital Health, Inc.
In addition, the system can measure a variety of physiologic parameters (heart rate, heart rate variability, activity, body position) on an ambulatory and periodic basis, and can aggregate at the database server level parallel physiologic metrics obtained with peripheral devices such as wireless blood pressure cuffs, glucometers, and weight scales. These combined data streams allow therapeutic events to be viewed in the context of a person’s physiology, thereby providing important links between
treatment, behavior, wellness, and therapeutic response.
The concept of biomedical telemetry from ingestible electronics was proposed in the 1960s in a series of lectures aimed at educating biologists, physicians, and engineers about
the use of radio to sense and transmit biological signals
from animals and humans [6].
The edible sensor has at its core a conventional silicon IC measuring 1.0 mm x 1.0 mm x 0.45 mm. The IC is coated with a copper salt on one side and magnesium on the other.
The edible sensor has at its core a conventional silicon IC measuring 1.0 mm x 1.0 mm x 0.45 mm. The IC is coated with a copper salt on one side and magnesium on the other.
The wearable health monitor serves to collect data from different sensors both internal and external to the body.
Wireless technology
allows data collected by the wearable health monitor and edible sensor to be instantly available throughout the spectrum of care. Ongoing efforts are being made to identify radio link innovations between the wearable health monitor and mobile phone including Bluetooth, BLE, Zigbee, and ANT.
Kit Yee Au-Yeung PATENT
https://patents.justia.com/patent/20180256108 US Patent Application for INSERTER AND METHOD OF INSERTING AN IMPLANT UNDER THE SKIN Patent Application (Application #20180256108 issued September 13, 2018) - Justia Patents Search
Mar 13, 2018
Some embodiments described herein relate to an apparatus, such as an inserter, having a housing and a needle at least partially disposed within the housing. An actuator can be coupled to the needle and configured to move the needle between an actuated configuration and a retracted configuration. A wire can be at least partially disposed within the needle. The wire can be fixedly and immovably coupled to the housing.
In some embodiments
a biosensor can be disposed within the needle
and the apparatus can be configured to deliver or implant the biosensor to a patient, for example, under the patient's skin.
The biosensor can constructed of a non-rigid material, such as hydrogel.
In some embodiments, the apparatus can be configured to deliver or implant the biosensor without applying a force to the biosensor.
CROSS-REFERENCE TO RELATED APPLICATIONS
An implantable biosensor can be implanted within the subcutaneous tissue space as well as within the layers of skin, intramuscularly or within the vasculature. Implanting the biosensor into these locations permits the sensing of biological parameters and/or biological analytes for both discrete and/or continuous monitoring.
The implantation process of such a miniaturized biosensors can be accomplished by injection through a conventional, medical-grade needle/syringe.
The wire can be fixedly coupled to the housing. In some embodiments, a biosensor can be disposed within the needle and the apparatus can be configured to deliver or implant the biosensor to a patient, for example, under the patient's skin.
The biosensor can constructed of a non-rigid material, such as hydrogel.
In some embodiments, the apparatus can be configured to deliver or implant the biosensor without applying a force to the biosensor.
Kit Yee Au-Yeung
https://www.patents-review.com/a/20190325734-mobile-communication-device-system-method.html
MONEY AND TECHNOLOGY
January 7 at 12:50 p.m., Hotel Adagio, the Ensemble Room:
George Savage, MD, Proteus Digital Health Chief Medical Officer and Co-Founder will present on a panel entitled
“From Digital/Physical Health to Behavior Change”
at the Annual Digital Health Luncheon at JPM, sponsored by W2O and Squire Patton Boggs.
January 9 at 2:00 p.m., San Francisco St. Regis Hotel, the Conservatory Room:
David O’Reilly, Proteus Digital Health Chief Platform Officer will present on a panel entitled “Digital Medicine: Lessons from Biopharma” at the Goodwin Speaks – Goodwin Seminars.
https://www.jpmorgan.com/solutions/cib/insights/healthcare-conference-highlights
The Conference is also the birthplace of a lot of deals, with companies announcing major transactions in the run-up.
The growing technological advancements and increasing expenditure in the healthcare industry are pushing the IoT (Internet of Things) in healthcare.






























Yeah great Summary. But there is more in Preparation.
https://julimination.wordpress.com/2023/09/19/why-are-the-world-economic-forum-wef-rockefeller-foundation-so-intent-on-implementing-a-digital-id-digital-currency-social-credit-score-system/
https://julimination.wordpress.com/2023/09/22/the-new-normal-aka-the-big-hack-of-life/
So this is a war of the upper 1% against the rest of us, means the own population!
How can we survive to such a evil and powerful murderous cartel?
Sometimes I wish to wake up from this nightmare...