https://www.francesoir.fr/societe-sante/lentreprise-gilead-aurait-elle-dissimule-la-vraie-toxicite-du-vekluryc-remdesivir Would Gilead have concealed the true toxicity of Veklury© (remdesivir)? | FranceSoir
The media has focused on the scripted binary debate around the use of hydroxychloroquine. Meanwhile, Veklury© (remdesivir) has been acclaimed to become the appropriate remedy despite its virtual therapeutic ineffectiveness.
In recent days, we learn that the United States would have seized the stocks of Veklury© and that the European Medicines Agency (EMA) gave a marketing authorization (MA) of this drug for severe forms of Covid-19, MA still to be validated by the member states.
We are very surprised by these decisions, for several reasons.
The effectiveness of Veklury© against Covid-19 has not been demonstrated and the only study to attribute a "modest" effectiveness concludes, haphazardly, that its use would allow a faster discharge from hospitalization of a few days, an evaluation criterion that was not included in the initial version of the protocol. We invite you to refer to our analysis of this study to assess for yourself the weakness of the conclusions.
It is customary to use antivirals at an early stage of viral infections, when they are most likely to be effective. Indeed, many doctors believe that in the very advanced stages of the disease, it is no longer the virus that affects the body but the immune reaction, and therefore a pure antiviral like Veklury© is not justified. The positioning of Veklury© in the advanced stage of the disease raises questions.
The toxicity of the molecule, GS-5734, and its metabolite GS-441524 has not been seriously studied and the data on its metabolism in the body are not only incomplete but also based on a poorly defined biological model, in any case incomplete. The supposed target is RNA polymerase without its principle of action being established. Yet the pharmacodynamics of this molecule identified since 2012 would have had plenty of time to be perfectly characterized since.
Since its creation, this drug has never proven real efficacy in humans,
regardless of the virus for which it has been tested: Ebola, SARS-CoV, MERS-CoV.
Worse, in each of these pandemics, hasty conclusions have been systematically published on its effectiveness and presented to the general public while based on studies more than dubious, if not hazardous.
For Ebola, for example, it was concluded to be effective after testing it on two patients who recovered from the infection, without ever having demonstrated that this was due to the administration of remdesivir. Unfortunately for the French, despite its glaring failure during the Ebola outbreak, Gilead's scientific deception seems to be surviving the test of time. Recall that the patent expires in October 2035, and that this molecule is now recommended by the European Medicines Agency (EMA) for therapeutic use against Covid-19.
The results of a study published on July 6 on 5 patients treated with remdesivir at the Bichat hospital report serious side effects. "This series of cases of five COVID-19 patients requiring intensive care for respiratory distress and treated with remdesivir, highlights the complexity of using remdesivir in these critically ill patients. Remdesivir was discontinued for side effects in four patients, including 2 elevations in ALT (3 to 5 N) and 2 renal impairments requiring dyalism." The results of the study can be found in the article entitled: Case study of the first five patients treated with COVID-19 with remdesivir in France. A shocking element is the publication date of this study on June 30, 2020 (date of online availability) while the patients were treated between January 24 and March 1, 2020.
Such information on the toxicity of a medicinal product and consequences should have been taken into account by the EMA.
We present an overall analysis of the toxicity data so as not to get lost in the details of the studies in the MA dossier that we nevertheless invite you to read. These elements are important but too often allow drug manufacturers to slicing up information, as we have highlighted the main informationin Annex I, as well as the gaps.
We, the citizens and patients of Europe, call for these shortcomings to be corrected.
1/ Analysis of evidence on the toxicity of remdesivir
A- "Official" data
When a drug starts to be used in humans, doctors have information about pre-clinical toxicity studies (in vitro and animal data). For more information, you can refer to our article on pre-clinical drug development (our article on pre-clinical developments)
From the first clinical studies, adverse events reported by patients and investigated by physicians are recorded. This information is recorded in a document called "investigator's brochure" which is updated as clinical studies progress before MA.
A drug can benefit from a Temporary Use Authorization (ATU) before MA, doctors must then have access to the safety information known at this stage.
Once the medicine is marketed, it is packaged with an information leaflet which, among other things, informs about potential adverse events. To date, this leaflet is not accessible for France.
The document that will serve as a package leaflet for hospital doctors is not yet available and therefore the only document that will serve as a package leaflet for side effects is today the document produced by the EMA that we have mentioned and partly analysed in Annex I.
Yet this drug, which has existed since 2015, has already been used in France, especially during the Covid-19 pandemic, and would benefit a priori from an ATU. His leaflet is not found, just as is not found his ATU sheet on the website of the ANSM (National Agency for the Safety of Medicines and Health Products). It seems that in the recent European MA dossier, not all known adverse events are reported by health agencies, since just for the first half of 2020 in France, there are at least 8 serious events reported on 22/06/2020, including 3 cases of renal failure requiring dialysis. As the sales figures of the product are not available, the risk analysis cannot be deduced, but knowing that the product has been little used in France, this already raises questions about these elements alone.
It should be noted that Gilead does not publish on its website any precise information on the side effects of the drug, let alone on its drug interactions. If the company or the doctors under agreement talk about the benefits of the product, no one mentions the side effects (press releases and other announcements on the financial markets). In addition, in its patent, Gilead repeatedly states the safety of the drug if it is properly dosed, from infinitesimal doses up to 100 mg/kg/day (1). It should be noted that the current mode of administration of this molecule is intravenous.
Official data on toxicity, side effects and drug interactions are therefore almost non-existent or very fragmented. It is strange to note that the document produced by the EMA, which repeats the one sent to the FDA (Food and Drug Administration, the American health authority) does not raise the question of toxicity in general, and more particularly of genotoxicity and mutagenicity, studies that should be available today for this molecule whose "first administration to human" is not new. But this is not the only element of toxicity of Veklury (remdesivir) that is obscured by the EMA document.
We are therefore surprised by the respective decisions of the international authorities, WHO, FDA and EMA, to initially authorize the use of Veklury© on a compassionate basis and recently as a treatment for Covid-19.
We referred to the FDA press release authorizing its use (2). Based on data provided by Gilead to the FDA, Veklury (remdesivir) would not pose a risk of toxicity to the body, except for rats dosed above 3 mg/kg/day. It would thus only be a question of contraindication or appropriate administration for certain patients suffering from renal or hepatic disorders.
Gilead and the EMA jointly claim that the toxicity of Veklury (remdesivir) is "acceptable" in view of the benefits, which so far, it must be admitted, are more than poor.
The only benefit really measured in phase III is indeed a reduction in the number of days of hospitalization from 15 to 11 for patients who would have recovered anyway, the mortality rate remaining the same with or without Veklury (remdesivir).
It should be remembered that the initial criterion of the study that came to these conclusions was precisely the mortality rate, and that if it had not been modified, the study would never have been able to conclude that it was less effective.
This could be consistent with the results of phase III trials in China and the United States that failed to demonstrate efficacy of Veklury (remdesivir) in patients of Asian or African descent.
As for efficacy, despite a very questionable attempt to make believe that the use of Veklury (remdesivir) could shorten the number of days in intensive care, all serious studies do not point to the beginning of efficacy, regardless of its use. And as France evening wrote, the pivotal study of the European MA dossier is also dubious.
It is urgent that European citizens demand a re-analysis of data and additional information. Evoking the benefit/risk dimension in the context is daring.
However, Gilead takes care to specify that precautions should be taken for patients with kidney and liver problems, or even advises against the use of Veklury (remdesivir) in some cases, this proves that the tolerance of the molecule is not harmless.
However, the toxicities indicated by Gilead are far from accounting for reality.
B- Research data
Searching the PubChem site of the National Center for Biotechnology Information (3) yields no information on the toxicity of Veklury (remdesivir) but the reader is referred via a link to a Canadian drug database, DrugBank (4). In this database, it is advisable to refer to a medicinal product with structural similarities, aciclovir, which is specified to have significant side effects up to acute encephalopathy (5a), or vidarabine (5b).)
The common element between these two molecules is an analogue group of nucleosides (flat conjugate group, nitrogenous base) that can potentially produce chromosomal damage in human cell cultures or be degraded into potentially toxic substances.
But what does the research say?
More and more cases of serious side effects of Veklury (remdesivir) are revealed around the world. This is how liver and kidney damage, but also cardiovascular problems have been reported and strangely little relayed by the media.
As FranceSoir points out, the American Society of Health-System Pharmacists, an American foundation that offers pharmacovigilance carried out by pharmacists themselves, is beginning to report alerts about the side effects observed (6). Indeed, Barbara F. Young, an editor of this foundation, pharmacist, explains that "the first data on the human safety of remdesivir came from the Ebola virus treatment system, where the nucleotide analogue and polymerase inhibitor drug had what one review called 'an acceptable safety profile'. Although it was not more effective than other experimental options tried.
The only adverse events reported in this trial were deaths, and the only one judged to be potentially attributable to remdesivir was hypotension followed promptly by cardiac arrest. »
The pharmacist adds about the adverse effects retained: "It was surprising when they came out; There was a very short list of side effects. It's either the safest drug there is, or... ». Or everything is done to conceal the toxicity of this drug. To be convinced, we must therefore review the tests carried out with Veklury (remdesivir). We therefore browse the publications of the dedicated site, PubChem of the National Center for Biotechnology Information (3). Again, despite the many clinical trials conducted since 2015, there are no precise and rigorous elements whereas normally phase I and II trials are precisely intended to evaluate the tolerance of a drug. It is noted that either the trials were stopped early for ill-defined reasons, or that it was considered that the damage was caused by the disease. In both cases, we would like to have a clear analysis of the conclusions of accountability of the responsibility of the molecule.
Another Chinese woman had reported side effects that would cause infertility in men (7).
This study was removed from the file without knowing why. It is surprising to see a preliminary study withdrawn, especially since the researchers were only making assumptions. And of course, the results of this study will be put into perspective of the following in the following paragraphs.
On the description of remdesivir, we note that toxicity is not assessed due to insufficient data. But in the "Safety and hazards" section, we are challenged by a pictogram that seems to evoke a fairly significant toxicity.
How is it that a molecule with such a serious warning about its impact on the body has not been the subject of further clinical safety studies?
To understand this paradox, we conducted a prospective analysis based on the chemistry of this molecule whose proprietary unique identifier code is GS-5734.
II/ Prospective toxicity analysis of Veklury (remdesivir).
To be able to consider the toxicity of this drug, we looked at the chemistry of the molecule, a natural line of investigation.
A- Biochemistry of Veklury (remdesivir).
Veklury (remdesivir) is an analogue of adenosine (8), a molecule used in cardiology whose pharmacodynamic effect is at the root of potential cardiac side effects, and also responsible for pulmonary adverse events (9).
Its short lifespan in the body (which is explained by the carbon-carbon bridge between the ribose part and the adenine part) generally makes it possible to quickly control this toxicity.
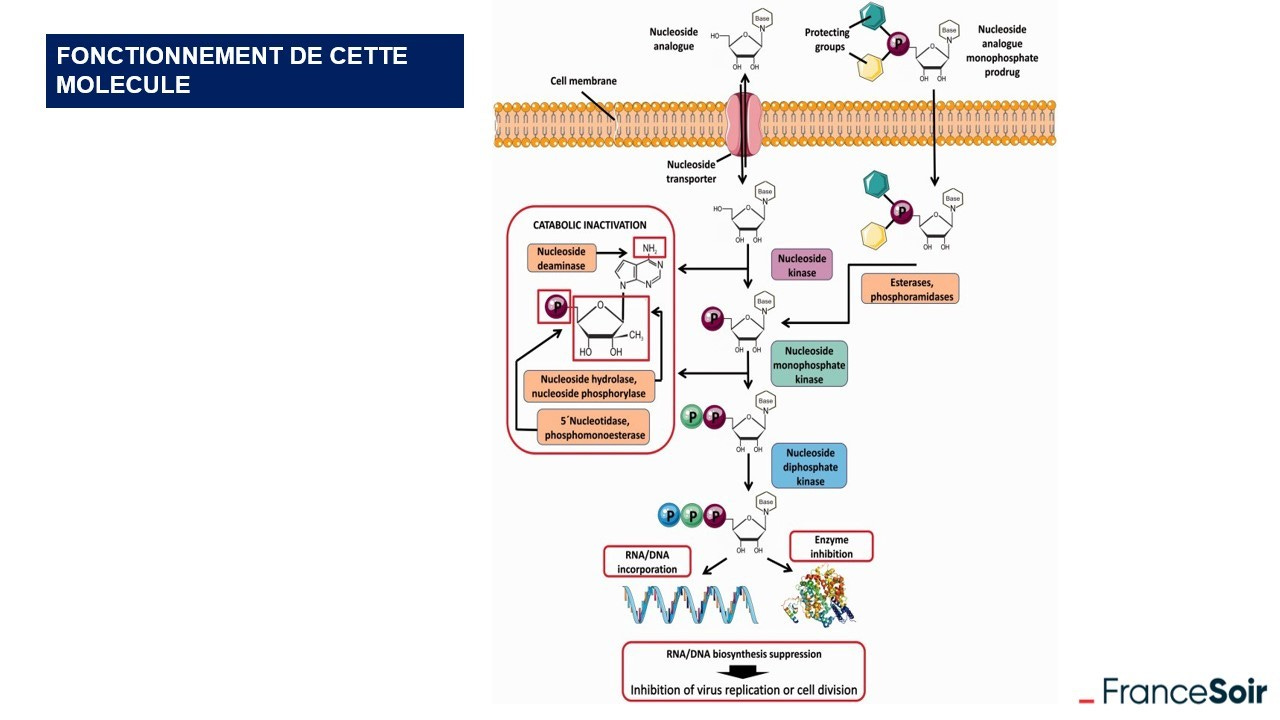
Gilead claims to have transformed this bridge to make the molecule more stable. It should be realized that Veklury (remdesivir) (GS-5734) is a prodrug, which means that once metabolized in the body, it will break down to give the active molecule, identified as GS-441524.
The chemists deliberately created a nucleoside analogue close to adenosine since the objective was precisely to be able to interact with the viral RNA in order to neutralize it and thus block replication. Gilead lays out simple models through numerous diagrams explaining how this drug works.
This diagram is only a model and only partially takes into account the many possible and even probable molecular interactions within the organism. It should be noted that this model was developed in vitro with isolated cells. However, the two states in which Veklury (remdesivir) will be found in the body suggest many interactions that raise questions about the simplified, if not simplistic, model that underlies the effectiveness of this drug. Indeed, these two molecules have chemical groups with high reactivity.
It is important to note that the prodrug, GS-5734, is accompanied intravenously by an adjuvant that serves as a vehicle, SBECD. It is a long oligosaccharide that can affect kidney and liver function.
Its manufacturer Gilead claims that the doses used are minute and the duration of exposure short (10). However, it is questionable whether some of the renal and hepatic damage observed in some patients is due to this SBECD.
The structure of the active molecule, GS-441524, is indeed very close to adenosine. There are, however, two fundamental differences. GS-441524 is much more stable than adenosine and therefore remains in the body longer. However, the toxicity of adenosine is formidable when the body is exposed to it durably, it is only the very short life of the molecule that makes its toxicity temporary and therefore tolerable. Heart, liver, kidney problems would otherwise be more than frequent in its therapeutic use. In addition, the mutagenic character of adenosine is very powerful, it is also the essence of the molecule since it is that it is incorporated into the viral RNA.
It is important to emphasize the mutagenic and carcinogenic nature of adenosine and, by extrapolation, the mutagenic and carcinogenic potential of GS-441524. Adenosine plays an important role in the development of many cancers, particularly through its immunosuppressive role in the antitumor response (11). So much so that it is one of the most promising avenues in cancer research. Unfortunately,
these effects could never be evaluated for Veklury©, the study on its long-term effects is absent from the pre-clinical record.
However, we can refer to the numerous studies on the toxicity of adenosine analogues and nucleosides, which speak volumes about the short- and long-term effects of this type of molecule (12).
Unless Gilead has found a magic formula, there is nothing in its current state to suggest that these toxic properties are absent for Veklury©.
The groups added to make the molecule more efficient have a high reactivity. Thus, to minimize this reactivity, the phosphate group was protected with esters and amides which, once in the cell, will be cleaved. What about the fate of these decompositions?
Another addition and not the least was made by the researchers to inhibit the interactions between mitochondrial RNA enzymes and the active molecule, interactions that killed the cell more than the virus. The Gilead chemists then admit to having tested several combinations to reduce this deleterious effect of the initial molecule and according to their tests, which we would like to be able to consult, it was the safest group. According to Katherine Seley-Radtke, Professor of Chemistry and Biochemistry and President-Elect of the International Society for Antiviral Research, "You can't predict activity. You have to do it and test it." He added: "But even small changes can have amazing consequences."
It should then be noted that this research was carried out in vivo, in the absence of many other elements that could precisely demonstrate the behavior of the molecule in the body. Thus, it is questionable whether this cyanide group has much more "surprising consequences" than the in vitro research presented by Gilead suggests. And in view of the reactivity and the many potential interactions, we can even ask if Veklury (remdesivir) really reaches its pharmaceutical target: SARS-CoV-2. It seems that for Ebola, SARS-CoV and MERS, its antiviral activity in vivo is speculative.
If Veklury© aka remdesivir does not reach the virus, it remains only its very high toxic potential as an impact on the body.
B- Veklury© or remdesivir is an altrononitrile.
Remdesivir is a nitrile. Specifically, an altrononitrile. This is described in its IUPAC name: l-alanine, N- ((S) -hydroxyphenoxyphosphinyl) -, 2-ethylbutyl ester, 6-ester with 2-C- (4-aminopyrrolo (2,1-f)(1,2,4) triazin-7-yl) -2,5-anhydro-d-altrononitrile.
Nitriles are very well known to chemists. These are very reactive and very often toxic molecules.
They are also used in the chemical industry to make insecticides, pesticides, powerful detergents for hard-to-strip materials such as metals.
Nitriles are cyaneous compounds.
This class of compounds is characterized by the presence of a C≡N group (cyano) and includes cyanides and nitriles (R–C≡N), as well as related chemical compounds such as cyanogens, isocyanates and cyanamides. They owe their toxicity mainly to the cyanide ion which, when released into the body, is able to inhibit many enzymes, especially cytochrome oxidase.
Death, which occurs more or less quickly depending on the rate of release of the cyanide ion, is the result of chemical asphyxiation at the cellular level.
The question that arises is the stability of this group within the molecule. Judging by the warning pictogram, nothing is less certain.
Nitriles or organic cyanides are therefore organic compounds characterized by the presence of a cyano functional group (–C≡N) whose general formula is RCN.
They can be considered as hydrocarbon derivatives in which three hydrogen atoms bonded to a primary carbon atom are replaced by a nitrilo group or by carboxylic acid derivatives (R–COOH) in which the oxo and hydroxyl radicals have been replaced by a nitrilo group (–C≡N).
By hydrolysis, they give an acid that contains the same number of carbon atoms and it is by this analogy that they are usually called hydrocyanic acid, rather than as derivatives of hydrogen cyanide. Nitriles are very dangerous compounds because they release hydrogen cyanide when they break down under the effect of heat.
Here are the types of symptoms that can cause a cyaneous agent:
The question is therefore whether our altrononitrile should be considered as the many toxic nitriles. Is Veklury (remdesivir) stable enough with respect to the nitrile group that it is not directly released or hydrolyzed to carboxylated acid residue?
What do we know about altrononitriles? Very little.
Cyan agents have a long military history.
Since the First World War, they have come in many forms and have proven to be formidable chemical weapons, such as Zyklon B.
C- Precaution for the manufacture of Veklury© or remdesivir
By referring to one of the prevention leaflets for the manufacture of GS-5734 and GS-441524 molecules, the two forms of remdesivir in the body, we see that
we are indeed dealing with a hypertoxic and corrosive nitrile,
which brings us back to the pictogram mentioned at the beginning of this article, which is present on the PubChem site of the National Center for Biotechnology Information (3).
Indeed, it can be seen that the warnings in the manufacturers' leaflets (14) warn about the significant toxicity of the product:
acute toxicity, skin corrosion/irritation, serious eye damage/irritation, respiratory or skin sensitisation, germ cell mutagen, reproductive toxicity, target organ specific toxicity (single exposure), target organ specific toxicity (repeated exposure).
We find absolutely all the potential attacks found in the most toxic nitriles, of which there is no need to recall their sinister use as combat gas.
If we look at the precautions to be taken when handling the products, we learn that leather should not be used because the product holes the leather and that rubber clothing must be washed immediately after use. We also learn that it is necessary to equip yourself with an oxygen mask because the product attacks the pulmonary epithelium, the same tissue that is preferentially seriously affected by Covid-19.
One element surprises us: there are risks of decomposition into various very toxic substances: carbon monoxide, carbon dioxide, nitrogen oxide (15).
For the active metabolite GS-441524, the manufacturer's package leaflets list the same acute toxicity elements (16).
Would the toxicity of remdesivir and its metabolite, GS-441524, stop on the surface of the skin? Skin that she would pierce if unfortunately a single drop was deposited there.
Gilead would contrast the low dosage (<3 mg/kg/day) and the short duration of exposure to minimize toxicity compared to the supposed benefit. However, there are no benefits, apart from a reduction of 4 days in the average hospitalization time.
No study today clearly allows to affirm that this drug would bring any other benefit.
It therefore appears that, in the light of the evidence presented above, we strongly believe that Veklury (remdesivir) is a harmful medicinal product and that these elements have been concealed by Gilead.
We believe that the lobbying operation conducted in the media and some public health authorities to discredit hydroxychloroquine, specifically in hospitals, was intended to make Veklury (remdesivir) the only solution in this situation.
We are still waiting for data and pharmacovigilance files for the first quarter of 2020 for these two molecules that the ANSM is resisting to produce.
Given the very high potential toxicity of remdesivir, we hypothesize that, despite its antiviral nature that would in principle make it suitable for early use, Gilead has so far only wanted to bring Veklury© to the market in the late phase of Covid-19.
The side effects and toxicity of the molecule can be "confused" with Covid-19 disease, it is very difficult to attribute to the use of Veklury©, the aggravation of respiratory distress, kidney or liver damage as well as cardiovascular complications.
Moreover, the phase III trial published in the NEJM carefully avoids giving the final mortality (at 28 days) in the Veklury (remdesivir) arm compared to placebo, which is more than abnormal. This essay is all the more questionable as it is almost impossible to find the figures corresponding to the data tables in the text. This study gives the impression of a scientific smokescreen in order seeking to drown the fish and find at all costs a form of result, while refraining from talking about the side effects (Appendix II).
In conclusion, it seems obvious to us that it is necessary to question officially the toxicity of this molecule with highly harmful properties and which has not demonstrated to date any patient benefit.
Appendix I: on toxicity
For example, the company should provide genotoxicity data that are referenced in the Compassionate Use Summary of Product Characteristics (SPC) provided to the FDA and in which it is mentioned very briefly that "remdesivir was not genotoxic in a battery of assays, including bacterial mutagenicity, chromosome aberration using human peripheral blood lymphocytes, and in vivo rat micronucleus assays", which translates to "Remdesivir did not demonstrate genotoxic effect in a series of tests performed, including a bacterial mutagenesis study, the detection of chromosomal aberrations in human lymphocytes, and in vivo studies in rats." It is strange to note that when it then arrives on the desk of the evaluator of the European Health Agency (EMA), remdesivir qualified as non-genotoxic and non-mutagenic becomes implicitly so to a very vague degree that remains to be defined. "A short discussion regarding assessment of mutagenic and potentially mutagenic impurities is presented and is acceptable considering the acute, potentially life-saving use. It is expected that this section is further extended for any upcoming applications. ", which translates to: "A brief discussion on the assessment of the mutagenicity of the product and potentially its impurities is presented and is acceptable considering the urgency of its potential life-saving use. It is expected that this section will be further developed for future recordings. ». This implies that Veklury (remdesivir) is intended to be used on a larger scale, or even on a very large scale, beyond compassionate care, which is obviously Gilead's goal. The document explains that the missing data on the genotoxicity and mutagenicity of remdesivir will be completed in due course. If the subject were not so serious, the semantic contortion of this document would be most delectable.
This type of procedure is devious because it makes it possible to sweep by the wayside, under the pretext of urgency, crucial elements on the assessment of the real toxicity of remdesivir.
Everyone has been able to realize in the course of life that postponing an essential task usually results in disaster. The more time passes, the less these elements will be brought to the attention of health authorities and therefore of attending physicians in hospitals.
Faced with such negligence, one is entitled to wonder if after years of use, harmful effects will eventually appear frequently enough to lead to yet another health scandal.
Who is Ms. Janet Koënig, the EMA rapporteur responsible for editing this document, which will be the basis of the blank check given to Veklury© (remdesivir), initially in compassionate use and then in a generalized way in the treatment of Covid-19?
Annex II
We asked EMA President Christa Wirthumer-Hoche for an interview in order to understand the Veklury© approval procedure in detail.
And the answer was that the president was not the right person to answer us because the molecule had been evaluated through a centralized procedure, and it was the CHMP that had given a favourable opinion.
We contacted the CHMP (Committee for Medicinal Products for Human Use), without success.
Annex III
FranceSoir analysis of the opinion of the European Medicines Agency
Review of the New England Journal of Medicine study on remdesivir
PLEASE LOOK AT THE RESULTS OF THESE STUDIES ON REMDESIVIR, IVERMECTIN, NIGELLA SATIVA, QUERCETIN, HCQ, MELATONIN, ETC.:
REMDESIVIR
https://c19early.org/smeta.html Remdesivir for COVID-19: real-time meta analysis of 57 studies (c19early.org)
IN COMPARISON, IVERMECTIN:
https://c19ivm.org/meta.html Ivermectin for COVID-19: real-time meta analysis of 99 studies
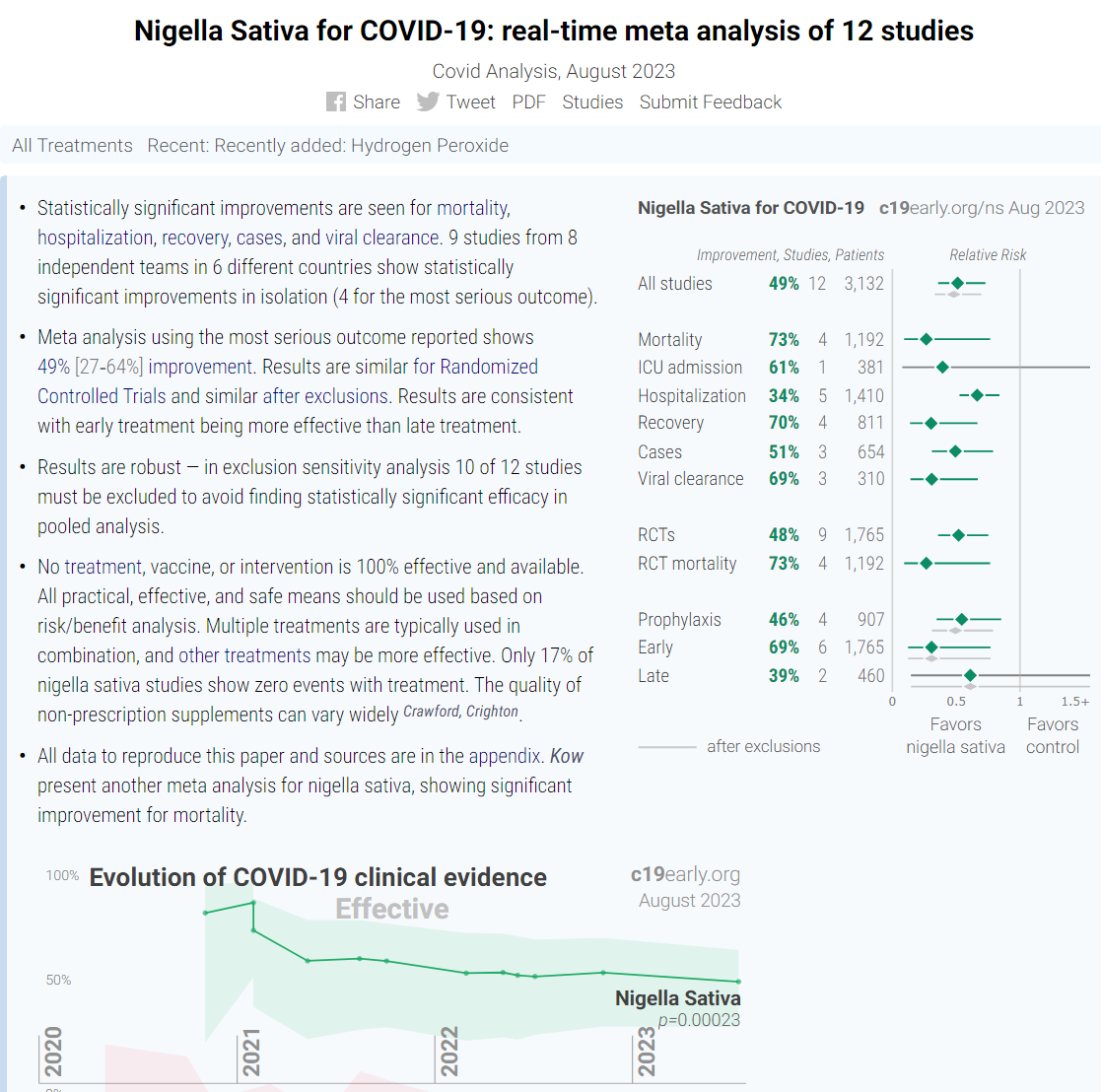
NIGELLA SATIVA:
https://c19early.org/nsmeta.html Nigella Sativa for COVID-19: real-time meta analysis of 12 studies (c19early.org)
OR QUERCETIN:
https://c19early.org/qmeta.html Quercetin for COVID-19: real-time meta analysis of 11 studies (c19early.org)
HYDROXYCHLOROQUINE
https://c19hcq.org/meta.html HCQ for COVID-19: real-time meta analysis of 408 studies (c19hcq.org)
MELATONIN
https://c19early.org/jmeta.html Melatonin for COVID-19: real-time meta analysis of 18 studies (c19early.org)
Yet, Remdesivir was the first drug to receive emergency use authorization from the U.S. Food and Drug Administration (FDA) to treat people hospitalized with C-19.
https://www.archyde.com/doctor-jean-paul-theron-arrested-by-the-gendarmes/
Posted on September 20, 2021 at 10:23 a.m.,
updated on September 20, 2021 at 11:11 a.m.
Doctor Jean-Paul Théron, referring doctor of the Manu iti center in Paea and supporter of treatments based on Ivermectin or Hydroxy chloroquine, has just been arrested by the gendarmes.
His daughter was quick to react:
Last Thursday, according to our colleagues from La Dépêche, the doctor would have injured the bailiff who came to his home to notify him of the filing of a complaint from the Country, instructed by the Council of the Order of Physicians … And yesterday, he has received a visit from the gendarmes. Jean-Paul Théron today evokes a false aggression.
https://en.wikipedia.org/wiki/Jean-Paul_Theron
(RECENT) Historic Ivermectin Court Victory in Malaysia
https://www.malaysiakini.com/news/676954 Court upholds doctors' right to dispense Ivermectin (malaysiakini.com)
MEANWHILE, THE WORLD HEALTH ORGANISATION PREPARES FOR THE NEXT ROUND OF REMDESIVIR USE
https://www.who.int/news-room/events/detail/2023/03/10/default-calendar/who-consultation---integrating-research-into-outbreaks--how-can-we-prepare-for-the-next-marburg-outbreak Integrating Research into Outbreaks: How can we prepare for the next Marburg outbreak? (who.int)
Session 4. Towards agreed trial platforms and simple protocols for therapeutics studies?
Candidate therapeutics developers’ update:
The treatment is usually 5 days and cost $3100 and up. Not to mention, under the CTAP program, hospitals are being financially incentivized for administering RMV to patients.
MY TAKE ON THIS?
ANOTHER NANOTECHNOLOGY INJECTION…
https://topclassactions.com/lawsuit-settlements/lawsuit-news/covid-19-class-action-lawsuit-and-settlement-news/remdesivir-recalled-by-gilead-after-glass-found-in-covid-19-drug/ Remdesivir Recalled by Gilead After Glass Found in COVID-19 Drug - Top Class Actions
The recall affects up to 55,000 vials, company spokesman Chris Ridley told Bloomberg. The affected product is Veklury (remdesivir 100mg for injection) with the FDA National Drug Code of 61958-2901-02. The affected lot numbers are:
2141001-1A, which was distributed nationwide to US wholesalers between 10/25/21 and 10/26/2021
2141002-1A, which was distributed nationwide to US wholesalers between 10/26/21 and 11/02/2021
Both lots carry an expiration date of January 2024.
https://www.drugs.com/sfx/veklury-side-effects.html#
https://childrenshealthdefense.org/defender/lawsuits-remdesivir-covid-cola/


























Dr. Brya Ardis, MD, also reveals more filth behind Veklury® (remdesivir), in this Principia Scientific article, where he notes Veklury “is a nucleotide analogue RNA polymerase inhibitor. Dr. Ardis reveals that the symptoms of lungs filling with fluid and the other alleged COVID-19 symptoms were actually side effects of kidney poisoning and other organ damage that are known side-effects of Veklury® (remdesivir). Dr. Ardis alleges that the devestating health toll allegedly caused by COVID-19 was actually caused by the NIH recommended treatment of Veklury® (remdesivir). Dr. Bryan states that the NIH even cited two studies on its website that showed that Veklury® (remdesivir) was ineffective and unsafe to patients. It seems that many doctors just blindly followed the recommendation of the NIH to use Veklury® (remdesivir) without actually reading the cited studies.” Dr. Ardis actually tracked down those studies and read them, and a complete list of adverse effects is listed in his article here. Or you can read yourself at the New England Journal of Medicine here. Don’t have time to read it? Ok, here, let me help:
And conflict of interest (follow the money they told us at Watergate)? The following is a list of those people on the NIH Panel on COVID-19 Treatment Guidelines who had financial ties to Gilead Sciences, the manufacturer of Veklury® (remdesivir):
Rajesh Gandhi is on the advisory board of Gilead Sciences.
David Glidden is a consultant for Gilead Sciences.
Adaora Adimora is a consultant for Gilead Sciences and received research support from Gilead Sciences.
Eric Daar is a consultant for Gilead Sciences and recieves research support from Gilead Sciences.
Judith Aberg received research support from Gilead Sciences.
Jason Baker received research support from Gilead Sciences.
Susanna Naggie received research support from Gilead Sciences.
Pablo Tebas received research support from Gilead Sciences.
Roger Bedimo received an honoraria from Gilead Sciences
People are so afraid of free radicals and oxidative stress, but this is why sunlight is so important - it creates free radicals in an acute way, then quells them with subcellular melatonin:
https://romanshapoval.substack.com/p/how-to-treat-emf-radiation-part-1
Love the compilation of info Mr. Human. Thank you.