Intrinsic Toxicity of Nanoparticles
Yes, there is a cause of both adverse effects and deaths:
the toxicity of nanotechnology.
https://medalerts.org/vaersdb/findfield.php?TABLE=ON&GROUP1=CAT&EVENTS=ON&VAX=COVID19 Search Results from the VAERS Database (medalerts.org)
COVID-19 MRNA VACCINE MODERNA ORIGINAL/OMICRON BA.1 (ELASOMERAN, IMELASOMERAN)
COVID-19 MRNA VACCINE MODERNA ORIGINAL/OMICRON BA.4-5 (ELASOMERAN, DAVESOMERAN)
COVID-19 MRNA VACCINE PFIZER-BIONTECH ORIGINAL/OMICRON BA.1 (TOZINAMERAN, RILTOZINAMERAN)
COVID-19 MRNA VACCINE PFIZER-BIONTECH ORIGINAL/OMICRON BA.4-5 (TOZINAMERAN, FAMTOZINAMERAN)
COVID-19 VACCINE VIDPREVTYN BETA
https://www.intechopen.com/chapters/40260 Nanoparticles Toxicity and Their Routes of Exposures | IntechOpen
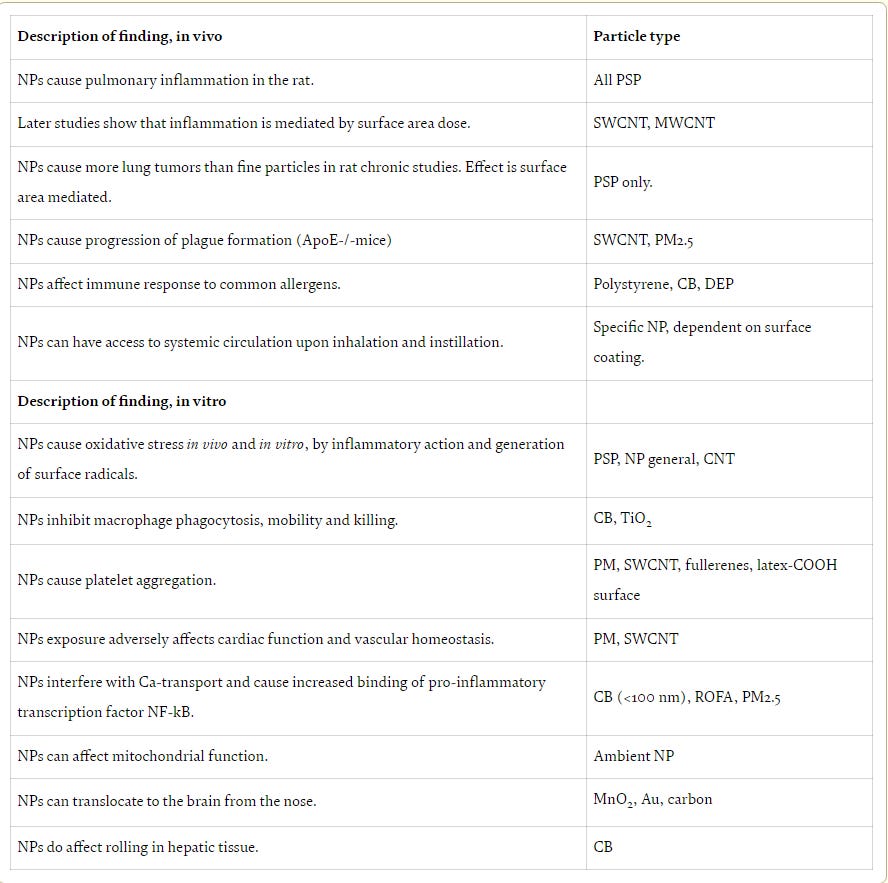
Nanoparticles have intrinsic toxicity profiles.
Properties of nanoparticles that might increase the toxicity potential include i) particle size, ii) surface area and charge, iii) shape/structure, iv) solubility, and v) surface coatings. Small size of NPs give rise to a high surface area per unit mass, and this surface area is often correlated with higher biological reactivity. In addition, formation of free radicals such as superoxide anion or hydroxyl radical may also be increased with high surface area. Accordingly oxidative stress may play an important role in NP toxicity especially for metal-based NPs. For example, inflammatory responses to NPs can be explained with these free radical formation.
2.2. Proposed mechanism of NP induced toxicity
2.2.1. Oxidative stress
Through many researches, reactive oxygene species (ROS) production is increased at NP exposure. This phenomenon is called oxidative stress. Knaapen et al. suggested three main factors which cause ROS release: (i) active redox cycling on the surface of NPs, particularly the metal-based NPs (ii) oxidative groups functionalized on NPs; and (iii) particle–cell interactions, especially in the lungs where there is a rich pool of ROS producers like the inflammatory phagocytes, neutrophils and macrophages. Overproduction of ROS activates cytokines and upregulates interleukin (IL), kinases and tumor necrosis factor-α (TNF-α) as an indicators of proinflammatory signaling processes as a counter reaction to oxidative stress.
2.2.2. Phagocytosis of NPs and inflammation
In respiratory tract, mucociliary clearance removes particulate matter (PM) in <6 µm diameter. Alveolar macrophages engulf and process particles that are not cleared by mucociliary action and coughing. Upon phagocytosis macrophages are activated to release substantial amounts of oxygen radicals, proteolytic enzymes, proinflammatory mediators and growth-regulating proteins. These mediators may lead to both acute and chronic lung inflammation.
As other NPs, the toxicity of Ag NPs appears to be driven by their oxidative and inflammatory nature, which then drives genotoxic and cytotoxic outcomes.
2.2.3. Genotoxicity
NanoGenotoxicology is yet another new term that was coined to represent the growing trend of research into NP-induced genotoxicity and carcinogenesis. Although there is still no exact correlation between NP-induced genotoxicity and lung cancer from epidemiological studies and in vivo rodent experiments, it is pointed out in literature that long-term inflammation and oxidative stress present in tissue can eventually induces DNA damage in cells and tissues.
Continuous ROS production in the cell can cause gene mutations/deletions leading to mutagenesis, carcinogenicity, and subsequently development of tumors and cancer.
As a result of DNA damage induced by NPs, single-strand DNA breaks, double-strand breaks, DNA deletions and genomic instability in the form of increase in 8-hydroxy-2-deoxyguanosine levels are formed.
The safety assessment of these new materials is mandatory to recognize risks and avoid potential hazards.
https://www.annualreviews.org/doi/10.1146/annurev-pharmtox-032320-110338 Nanoparticle Toxicology | Annual Review of Pharmacology and Toxicology (annualreviews.org)
NANOTOXICITY MECHANISMS
There are a number of different pathways by which nanoparticles can enter the body. These pathways include inhalation, oral ingestion, ocular exposure, application and deposition on skin, and intravenous administration.
The inhalation of airborne nanoparticles is a major exposure pathway that allows nanoparticles to enter and deposit in lung tissues and the alveolar region. Accumulation of nanoparticles in the lung can lead to oxidative stress–mediated lung inflammation at both acute and chronic stages. Inhalation may also lead to the accumulation of nanoparticles in the brain. Maher et al. reported that airborne magnetite nanoparticles can enter the brain via the olfactory bulb. [masks, PCR tests instillation] Accumulation of magnetite nanoparticles in the brain that are abundant in airborne particulate matter pollution can lead to enhanced production of ROS [Reactive Oxygen Species], which is causally linked to neurodegenerative diseases such as Alzheimer's disease.
After entering the body, nanoparticles may interact with the initially encountered organ or tissue. Nanoparticles may also subsequently translocate and enter the bloodstream (for example, from lungs to the capillary network to bigger vessels) to access distant organs/tissues via systemic transport. Within organs, tissues, and blood, nanoparticles can interact with cells and intracellular organelles to potentially cause toxicity at cellular and subcellular levels. It is important to point out that, upon entry into the body, nanoparticles interact with a variety of different biomolecules, including proteins, carbohydrates, lipids, and nucleic acids. These interactions result in the formation of a biomolecular nanoparticle surface corona, often referred to as the protein corona. Protein corona (or biomolecular corona) formation may change nanoparticle surface chemistry or stimulate the complement system substantially and ultimately affect nanotoxicity or the efficacy of nanomedicine. The damage of proteins to nanoparticle surfaces may also lead to protein unfolding [Prions - A prion is composed of protein in a misfolded form]. This process may induce the loss of protein function and may cause immunotoxicity. In addition, the protein configuration change can lead to adverse effects and toxicity via cell signaling pathway activation, enzyme function loss, nanoparticle aggregation, new antigenic site formation, and protein fibrillation [Protein fibrillation is involved in many human diseases, including Alzheimer's, Creutzfeld-Jacob disease, and dialysis-related amyloidosis. Protein amyloid fibrils have widespread implications for human health.].
At the cellular level, direct interaction between nanoparticles and cells may result in physical damage of cell membrane structures.
For example, graphene nanoparticles have been reported to cause physical damage, cytoskeletal dysfunction, and abnormal morphological stretching in different cell types as a result of the blade-like shape of these materials.
In addition, nanoparticles may be able to block cell membrane receptors and membrane ion channels, which may interrupt normal cellular biofunctions and homeostasis. Leifert et al. reported that 1.4-nm gold nanoparticles were able to block voltage-gated potassium channels in vitro, which may lead to unwanted cardiac malformation in mice.
A major nanotoxicity mechanism is the generation of ROS such as singlet oxygen, superoxide anion radicals, oxygen radicals, peroxide ions, hydrogen peroxide, and hydroxyl radicals. ROS generation can occur in different ways. One route is through one-electron oxidative reactions with transition metals or nanoparticle surface groups. It is important to note that a nanoparticle exhibits a relatively large surface area compared to the particle volume. An increase in surface area is typically accompanied by an increase in chemical reactivity potentially leading to increased ROS production. Another ROS generation mechanism is via mitochondrial respiration and subsequent ROS release into the cytoplasm through pores in mitochondrial membranes created by nanoparticles. In healthy cells, an equilibrium is maintained between intracellular antioxidants and ROS. However, intracellular nanoparticles can directly damage mitochondria, causing an increase in intracellular ROS and oxidative stress. Enhanced intracellular ROS levels may stimulate further ROS release from mitochondria through a process called ROS-induced ROS release. This process can substantially increase intracellular ROS levels and amplify the oxidative imbalance. High levels of ROS can cause oxidative stress and damage to cellular organelles, DNA, cell membranes, ion channels, and cell surface receptors, leading to adverse effects and toxicity. [https://outraged.substack.com/p/treatment-in-practice , https://outraged.substack.com/p/problem-and-solution]
Metal or metal oxide nanoparticles are used in preclinical and clinical applications such as imaging, photothermal therapy, and biosensors. However, corrosive tissue microenvironments and lysosomal degradation may disintegrate nanoparticles to release potentially harmful metal ions. For many nanoparticles, including Ag, cadmium selenide (CdSe), ZnO, and ferrosoferric oxide nanoparticles, released metal ions may generate high levels of oxidative stress and are primary sources of nanotoxicity. We want to emphasize that nanotoxicity results obtained in cell culture studies do not necessarily recapitulate the nanoparticle toxicity potential in animal models or human subjects. For example, CdSe quantum dots were found to be toxic in cell culture; however, no toxicity was observed in animal models under the specific reported testing conditions.
The generation of high levels of ROS and the release of harmful metal ions from nanoparticles have been reported to affect a variety of cell signaling pathways such as nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB), mitogen-activated protein kinase (MAPK), Akt, and Src. Activation and modulation of these signaling pathways can affect cell proliferation, differentiation, and cell survival. Nyga et al. reported that cobalt nanoparticles can stabilize hypoxia-inducible factor (HIF) protein and upregulate HIF gene expression in human macrophages. HIF pathway activation can affect cell growth, cell survival, apoptosis, and metabolic adaptation. Importantly, there can be interplay and synergistic effects between ROS generation, cell signaling modulation, and nanoparticle disintegration. For example, nanoparticle disintegration may lead to modulation of signaling pathways and/or induce ROS generation, which can activate numerous signaling pathways or cause nanoparticle disintegration; in turn, different cell signaling pathways can subsequently induce ROS generation. These mechanisms may cause damage to cell membranes, intracellular organelles, and nucleic acids and eventually lead to cell apoptosis or necrosis.
Loss of functional cells may compromise organ function and result in organ damage or inflammatory responses.
Moreover, cell apoptosis, necrosis, and pyroptosis may lead to the release of large amounts of intracellular content to potentially cause local inflammation or systemic immune responses.
In addition to the nanoparticle core material, surface components may also contribute significantly to nanoparticle adverse effects and toxicity. For example, researchers coat nanoparticle surfaces with polymers such as dextran or poly(ethylene glycol) (PEG) to reduce adsorption of proteins and other biomolecules and to prolong nanoparticle blood circulation times. Therefore, PEG is widely used in preclinical and clinical studies for surface modification of nanomedicines.
However, PEG may induce hypersensitivity reactions and anaphylaxis mediated by anti-PEG antibodies in humans.
After an initial systemic administration of PEGylated nanoparticles, anti-PEG immunoglobulins (Igs; IgM initially, then IgG) may be generated by marginal zone spleen B cells. The anti-PEG IgM then targets PEGylated nanoparticles during subsequent administrations, causing complement activation via the classical pathway. Upon activation of the complement system, anaphylatoxins will be released, including platelet-activating factor, histamine, or cytokines, resulting in hypersensitivity reactions. Kozma et al. documented the causal relationship between complement activation by anti-PEG IgM and hypersensitivity reactions in pig models. Although the study was conducted in pigs, it provides valuable insights into potential PEG-related toxicity mechanisms in humans. Other reported studies indicate that hypersensitivity to nanoparticle surface components may be induced by complement activation via the alternative pathway. This pathway does not depend on anti-PEG antibodies and is not limited to PEGylated nanoparticles. More in-depth studies are needed to fully elucidate the underlying mechanisms of hypersensitivity reactions. In addition to complement activation–related pseudoallergy reactions, complement-independent pseudoallergy is caused by anti-PEG IgG. Mechanistically, a PEGylated nanoparticle is bound by anti-PEG IgG forming the nanoparticle-IgG complex, which subsequently can interact with the Fcγ receptors on mast cells, basophils, and neutrophils, resulting in the release of platelet-activating factor, histamine, or cytokines, to induce hypersensitivity reactions.
Hypersensitivity reactions often occur during second- and later-stage administration of PEGylated nanoparticles. However, hypersensitivity reactions have also been observed during the first dosage in human subjects. A potential rationale for this side effect is the abundance of preexisting anti-PEG antibodies in some of the patient population. Many humans who had never received PEGylated materials or drugs still possess preexisting anti-PEG IgM and IgG in various amounts, which is likely due to exposure to PEG-containing over-the-counter medication (e.g., daily multigram doses in some laxatives), cosmetics, and other everyday consumer products.
According to a study by Yang et al., anti-PEG antibodies were detected in 72% of human samples collected after 1999, while 56% of historical samples from the previous 30 years (1970–1999) exhibited anti-PEG antibodies.
In other words, a large number of humans exhibit detectable levels of anti-PEG IgG and IgM, and these numbers are expected to increase in humans in the future as a result of wider exposure to products that contain PEG.
Besides hypersensitivity reactions, complement activation can also lead to a direct attack of the lipid membrane of drug-carrying nanoparticles, such as doxorubicin liposomes, to prematurely release encapsulated chemotherapy drugs. Such premature drug release can affect the therapeutic effect of nanomedicines and could potentially contribute to additional nanotoxicity concerns. Anti-PEG immunity may also contribute to the so-called accelerated blood clearance (ABC) phenomenon. Upon repeated administration of PEGylated nanoparticles, anti-PEG IgM opsonization may trigger efficient nanoparticle phagocytosis. As a result, nanoparticles may accumulate to a large extent in cells and organs of the mononuclear phagocyte system, including the liver, after their first administration, and thus not arrive at the intended target location such as a tumor. Besides the observed decrease in nanoparticle therapeutic efficacy upon ABC, acute and chronic nanotoxicity of sequestered nanoparticles are substantial concerns.








Any suggestions in terms of detox or solutions? Otherwise, amazing information! Thank you Mr. Human.
This was an interesting read. I’m not in the medical field so I had some new vocabulary words to look up. I have a family member with vax-induced ALS and a 21 year old daughter with post-Covid severe POTS. She is the only family member who never tested positive, but had the most Covid tests (allergies read the same as Covid symptoms, so...).
I wish we had more solutions to these devastating illnesses.