Vaxx, Bluetooth & directed energy tracking system
WEF, Bill Gates/Proteus and Co.
MUST-READ:
The World Economic Forum and Proteus, a Bill Gates company that manufactures a Bluetooth-enabled microchip/tracking device, have been cooperating for a long time.
Gate’s Proteus company Bluetooth-enable microchip patented as Ingestible therapy activator system and method, United States Patent 8,036,748 Zdeblick, et al.October 11, 2011
https://web.archive.org/web/20160730061918/http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=8,036,748.PN.&OS=PN/8,036,748&RS=PN/8,036,748
is connected with the following patent: Target tracking system and method with jitter reduction suitable for directed energy systems US20120104282A1 (US20120104282A1 - https://patents.google.com/patent/US20120104282A1/en)
https://patents.google.com/patent/US20120104282A1/en US20120104282A1 - Target tracking system and method with jitter reduction suitable for directed energy systems - Google Patents
United States Patent 8,036,748 Zdeblick , et al.October 11, 2011
Ingestible therapy activator system and method
Abstract
An ingestible therapy activator system and method are provided. In one aspect, the ingestible therapy activator includes an ingestible device having an effector module to send an effector instruction and a responder module associated with a therapeutic device. The responder module may receive and process the effector instruction, resulting in a response by the therapeutic device. Examples of responses by therapeutic device include activating a therapy, deactivating a therapy, modulating a therapy, and discontinuing a therapy.
Inventors: Zdeblick; Mark (Portola Valley, CA), Jensen; Marc (Los Gatos, CA), Colliou; Olivier (Los Gatos, CA), Strand; Angela (San Francisco, CA)
Assignee: Proteus Biomedical, Inc. (Redwood City, CA)
Family ID:42170749Appl. No.:12/665,111
Filed: November 13, 2009PCT
Filed: November 13, 2009PCT No.: PCT/US2009/064472371(c)(1),(2),(4) Date: December 17, 2009PCT Pub. No.:WO2010/057049PCT Pub. Date: May 20, 2010
Prior Publication Data
Document Identifier Publication Date US 20100312228
A1Dec 9, 2010 Related U.S. Patent Documents Application Number Filing Date Patent Number Issue Date 61114442 Nov 13, 2008
PATENT US 20100312228:
https://patents.google.com/patent/US20120104282A1/en
Target tracking system and method with jitter reduction suitable for directed energy systems
Abstract
Embodiments of a target tracking system and method with jitter reduction suitable for directed energy systems are generally described herein. In some embodiments, the directed energy system includes a target tracking system to track one or more track points on a moving target, and a beam transmission unit to maintain a directed energy beam on a selected one of the track points in response to tracking control signals provided by the target tracking system. The track points may be smaller than a spot size of the directed energy beam maintained on the target.
Proteus Biomedical:
Five among 34 worldwide honored as likely to have an impact on the future by the World Economic Forum
That futuristic combination of biomedical and electronic technology is not science fiction, though it's not yet on the market. The message-sending pill is the invention of Proteus Biomedical of Redwood City, one of five Bay area companies included among the 34 named Technology Pioneers 2009 by the World Economic Forum.
The independent organization based in Geneva recognizes "visionary companies" it deems likely to shape the future of business and society.
Proteus Biomedical envisions a future when drugs and medical devices do double duty. Beyond their core role as therapies, with the addition of a tiny microsensor, a drug capsule or pacemaker, could become a scout inside the body dispatching data back to patients and doctors.
Tracking compliance
Pills made with this "intelligent medicine" approach can report exactly when they've been swallowed. Proteus chief executive Andrew Thompson said this could help patients keep track of their compliance with treatment schedules, which can be crucial for staving off serious health damage such as heart failure. Avoiding unnecessary hospital admissions also could slash national health care expenditures, he said. "It costs the system a fortune."
How The Platform Works
Using a Bluetooth-enabled device – like the one you already carry in your pocket or purse – you can access secure applications that display your data in context and support care in a variety of different ways.
What is claimed is:
1. A system for providing instructions to a therapeutic device, the system comprising: an ingestible unit comprising: a housing; and an effector module secured within the housing for providing an effector instruction, wherein the housing dissolves upon contact with the surrounding fluid of a desired target site to release the effector module and the effector module comprises a support structure including two dissimilar materials deposited thereon wherein the dissimilar materials represent a voltage potential difference and provide power for the ingestible unit upon contact with the fluid; a responder module in communication with the therapeutic device for receiving and processing the effector instruction, wherein the effector instruction alters at least an operation of the therapeutic device;
and a hermetically sealed conductance control module electrically coupled to each of the dissimilar materials for controlling the conductance between the dissimilar materials to generate a unique current signature that presents the effector instruction, wherein the current signature is produced through controlled ionic emission.
2. The system of claim 1, wherein the ingestible unit further comprises an oral medication.
3. The system of claim 1, wherein the ingestible unit further comprises a second effector module secured within the housing to provide a second effector instruction.
4. The system of claim 1, wherein the effector instruction further comprises data.
5. The system of claim 1, wherein the responder module further comprises a transmitter to transmit at least one of data and signals.
6. The system of claim 1, further comprising the therapeutic device.
7. The system of claim 6, wherein the therapeutic device is a cardiac device.
8. The system of claim 7, wherein the cardiac device is a lead device.
9. The system of claim 6, wherein the therapeutic device is a selected from a group consisting essentially of an electrode device, a migraine device, a urinary device, and a gastrointestinal device.
10. The system of claim 1, wherein the responder module comprises at least one of a hardware component and a software component.
Description
FIELD OF THE INVENTION
The present invention relates generally to medical therapy systems, devices, and methods. More specifically, the invention relates to systems, devices, and methods for activation and/or modulation of various medical therapies using an ingestible
electronic device.
BACKGROUND
Multiple therapies exist for various health-related conditions, events, and defects.
Such therapies may be implemented as implanted devices, e.g., cardiac rhythm management devices, neural stimulation/neuromodulation devices, intrathecal drug delivery pumps, and functional neuromodulation prostheses such as cochlear implants, retinal implants, and artificial joints, limbs and organs. Once implanted, however, such devices may not provide the functionality to facilitate controlled activation or modification.
In one example, a neural stimulation device may deliver pain-control therapies to the spinal column, yet the neural stimulation device may not be activated on demand. Stated differently, the neural stimulation device is always activated after implantation. As a result, the patient may be subjected to neural stimulation, as well as its associated unwanted side effects, at times when such therapy is not needed.
In another example, a neural modulation device such as a spinal cord stimulator may provide therapeutic benefit for pain, yet the rate of stimulation may not be adjustable to align stimulation to the patient's lifestyle, e.g., a higher intensity during high activity periods and a lower intensity during low activity periods. As a result, the patient may need to restrict activities which do not conform to the stimulation intensity to receive optimal therapy results or suffer diminished therapy results when engaged in non-conforming activities.
Therefore, it would be desirable to have systems, devices, and methods for controlling therapies, for optimizing therapy results, and for enhancing patient treatment without having limitations placed on the patient.
SUMMARY
The present disclosure includes a system for providing instructions to a therapeutic device. The therapeutic device can be any type of device, such as a cardiac therapeutic device, a neural stimulation device, an intrathecal drug delivery pump, a gastrointestinal device; and a neural stimulation prosthesis. The system includes an ingestible unit and a responder module. The ingestible unit includes an output or effector module that provides an effector instruction to the responder module. The responder module receives and processes the effector instruction and communicates the effector instruction to the therapeutic device to alter the operation of the therapeutic device.
Certain aspects may be directed to in-body therapies and may include, for example, implantable medical devices. The term "implantable medical device", as used herein, refers to a device configured to be positioned at least partially on a living body, at least partially in a living body, or a combination thereof.
For example, the implantable medical device may include a lead having various electrode configurations communicably associated with controller circuitry, a power source, etc. More particularly and illustratively, the implantable medical device may comprise one or more leads with multiple in-line segmented electrode satellites, wherein each electrode is independently controllable and power/data wire(s) for multiplexing the multiple segmented electrode satellites. Various configurations of devices which may be used in conjunction with this invention may be described/disclosed in the PCT application no. PCT/US2003/039524 published as WO 2004/052182; PCT application No. PCT/US2005/031559 published as WO 2006/029090; PCT application No. PCT/US2005/046811 published as WO 2006/069322; PCT application No. PCT/US2005/046815 published as WO 2006/069323; PCT application No. PCT US2006/048944 published as WO 2007/075974; U.S. application Ser. No. 11/939,524 published as US 2008-0114230 A1.
Various configurations of devices which may be used in conjunction with this invention may be described/disclosed in PCT application Ser. Nos. PCT/US2008/052845 published as WO/2008/095183 and PCT/US2006/016370 published as WO/2006/116718. Each of the aforementioned applications is herein incorporated by reference in its entirety. The aforementioned configurations are for illustrative purposes only and that various other components and configurations are possible.
An Ionic Emission Module or Ingestible Event Marker (IEM) system described in PCT/US2008/52845, filed Feb. 1, 2008, and U.S. patent application Ser. No. 12/564,017, filed Sep. 21, 2009 (both of which are incorporated herein by reference) include an IEM and a personal signal receiver. Aspects of the IEM include an identifier, which may or may not be present in a physiologically acceptable carrier. The identifier is characterized by being activated upon contact with a target internal physiological site of a body, such as digestive tract internal target site. The personal signal receiver is configured to be associated with a physiological location, e.g., inside of or on the body, and to receive a signal of the IEM. During use, the IEM broadcasts a signal which is received by the personal signal receiver.
The IEM also includes two dissimilar materials deposited on two sides of the IEM to form electrochemical potentials and act as the cathode and the anode to form a power source. The dissimilar materials may be separated by a non-conducting material or skirt that amplifies the signal through increasing the current path. More specifically, the dissimilar materials can be made of any two materials appropriate for the environment in which the IEM will be operating. For example, when used with the ingestible device the dissimilar materials may be any pair of materials with different electrochemical potentials that are ingestible. An illustrative example includes the instance when the IEM is in contact with an ionic solution such as stomach acids, as shown in FIG. 8 below. Suitable materials are not restricted to metals, and in certain aspects the paired materials are chosen from metals and non-metals, e.g., a pair made up of a metal (such as Mg) and a salt (such as CuCl or CuI). With respect to the active electrode materials, any pairing of substances--metals, salts, or intercalation compounds--with suitably different electrochemical potentials (voltage) and low interfacial resistance are suitable. Additionally, a control module (not shown) is electrically coupled to each of the two dissimilar materials in order to receive power and become activated as well control the conductance and hence the current path between the two dissimilar material. The control module alters conductance between the dissimilar materials in a unique manner. By altering the conductance path between the dissimilar materials, the control module is capable of controlling the magnitude of the current through the conducting fluid/liquid that surrounds the IEM or ingestible device 200. This produces a unique current signature that carries or encodes the effector instruction and can be detected and measured by a receiver, such as the responder module 206, which can be positioned internal or external to the body.
Patent US 20100312228 A1
https://patents.google.com/patent/US20120104282A1/en US20120104282A1 - Target tracking system and method with jitter reduction suitable for directed energy systems - Google Patents
You got it RIGHT it is a tracking device or sensor for a target for directed energy weapon system
This application claims the benefit of U.S. Provisional Application No. 61/056,905, filed May 29, 2008 entitled APPARATUS AND METHODS FOR TRACKING SYSTEM FOR DIRECTED ENERGY WEAPONS.
[0001] Embodiments pertain to target tracking systems. Some embodiments pertain to directed energy systems (DES). Some embodiments pertain to active denial systems (ADS).
[0002] Some directed energy systems use a high power energy beam to counter threats instead of the chemical and kinetic energy used by more conventional weapons. Some directed energy systems, referred to as active denial systems, repel aggressors with a high-power energy beam, avoiding deadly force and causing collateral damage. One issue with these directed energy systems is accurately aiming the high-power energy beam on a particular target area long enough to cause a deterring effect. The larger the area on the target area that is radiated, the more energy that is needed to cause a deterring effect. Achieving a deterring effect is particularly difficult with a moving target because the target's movement and jitter effectively spreads the high-power energy beam over a larger target area, significantly increasing the amount of energy and/or time needed to cause a deterring effect.
[0003] Thus, there are general needs for target tracking systems that allow directed energy systems to reduce the amount of energy and/or time needed to cause a deterring effect on a moving target. There are also general needs for tracking systems for use with directed energy systems that can reduce and/or remove the effects of target movement and jitter. There are also general needs for directed energy systems that can use lower energy levels, which allow for the use of cost-effective solid-state amplifier components.
…
BRIEF DESCRIPTION OF THE DRAWINGS
[0005]
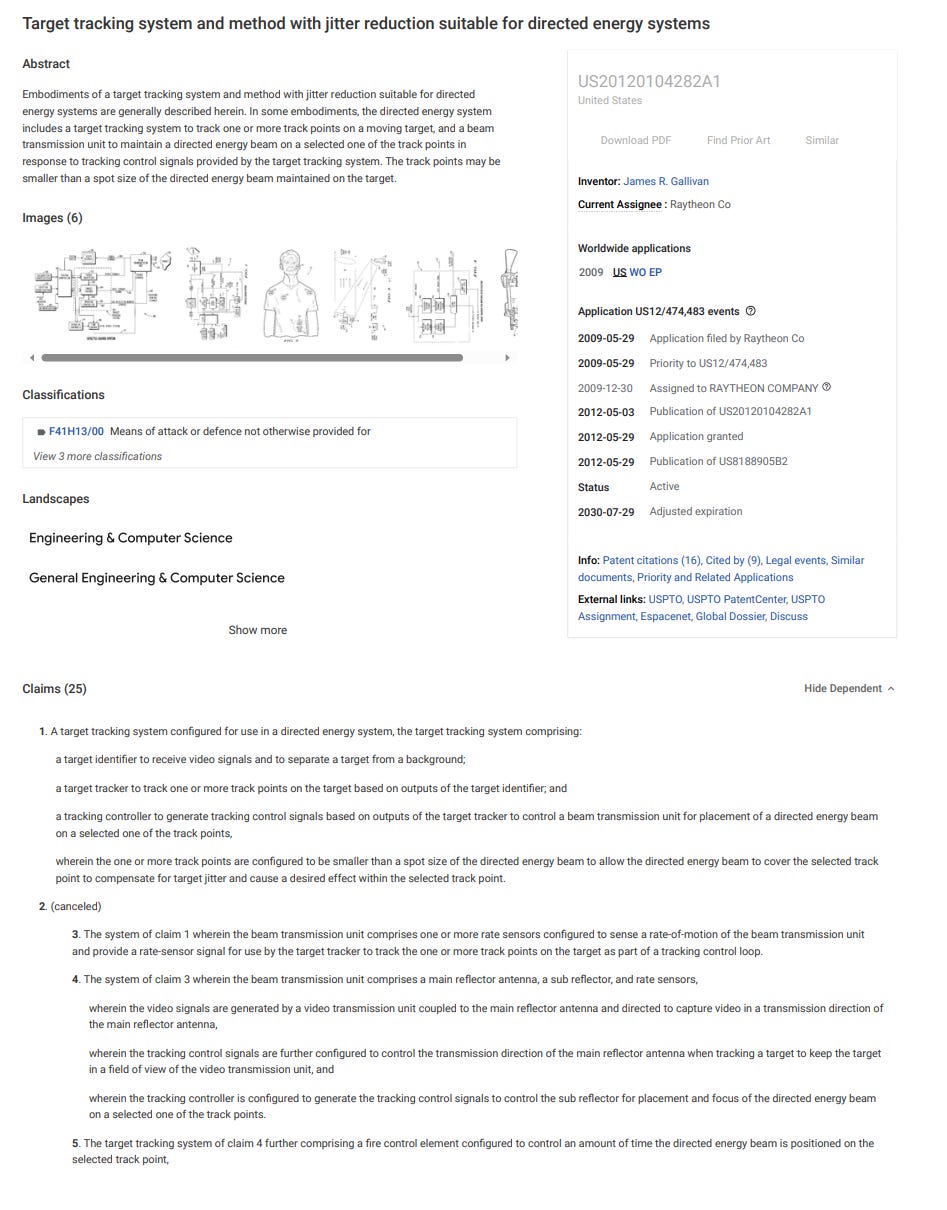
FIG. 1 is a functional diagram of a directed energy system, including a target tracking system, in accordance with some embodiments;
[0006]
FIG. 2 illustrates track points on an example target in accordance with some embodiments;
[0007]
FIG. 3 is an illustrative diagram of a beam transmission unit in accordance with some embodiments;
[0008]
FIG. 4 is a functional block diagram of a target tracker in accordance with some embodiments; and
[0009]
FIG. 5 illustrates an example hand-held directed energy system that includes a target tracking system in accordance with some embodiments.
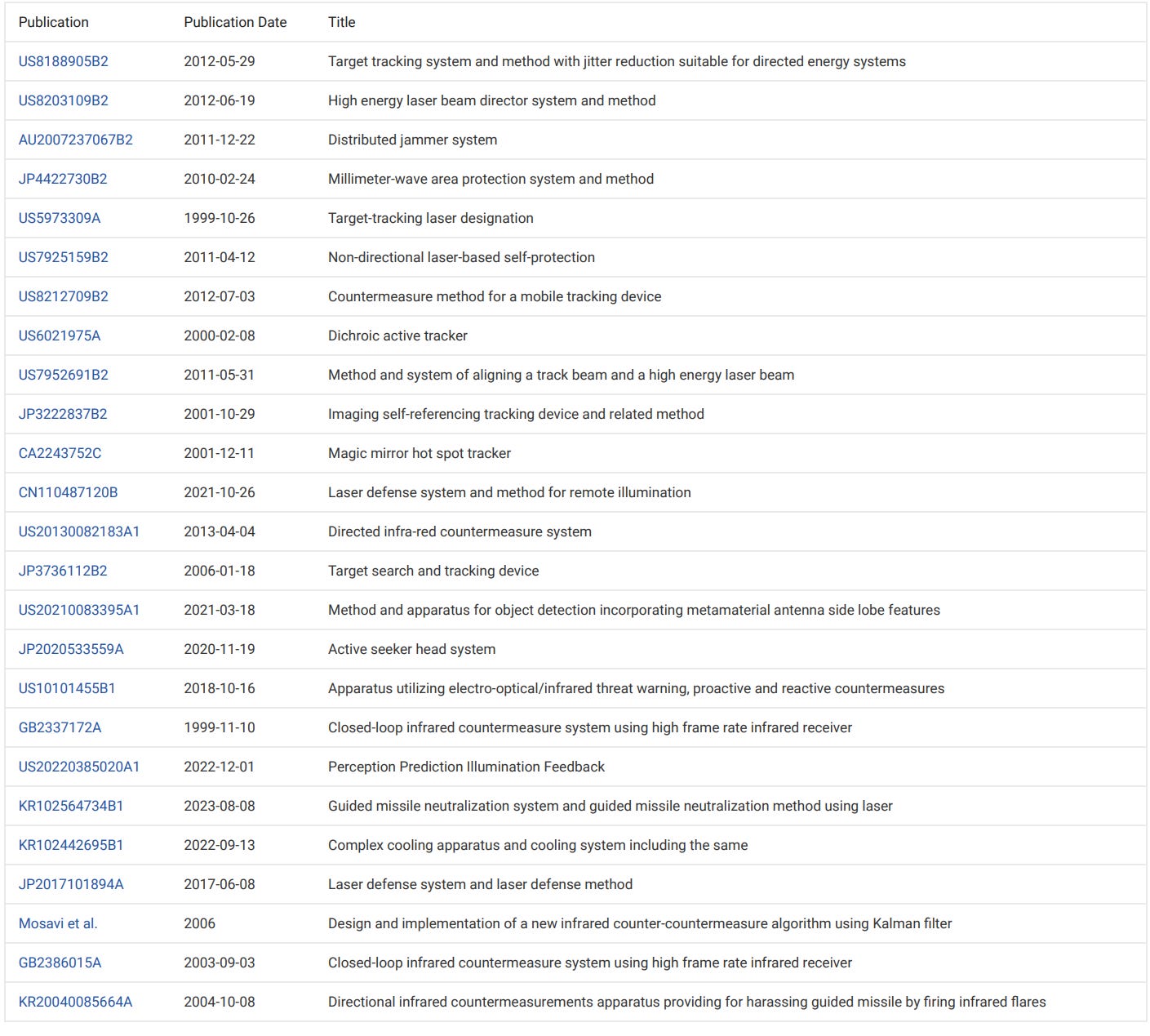
Similar Documents
https://web.archive.org/web/20150217024815/http://www.proteus.com/company/investors
https://web.archive.org/web/20050209193919/http://www.proteusbiomedical.com/bod.htm
Board of Directors
Frank Fischer
President & CEO, NeuropaceTerry Gould
Head of Direct Investments, Adam Street PartnersAlan J. Levy, Ph.D.
President & CEO, Northstar NeuroscienceBen Pless
CTO, NeuropaceRyan Schwarz
Principal, The Carlyle GroupAndrew Thompson
Proteus Biomedical Co-Founder, President & Chief Executive OfficerMark Zdeblick, Ph.D.
Proteus Biomedical Co-Founder, Chief Technology Officer
https://web.archive.org/web/20090210071204/http://proteus.swiftmob.com/quickPage.html?page=444
2ergo News
31 July 2008
2ergo Secures Deal with Major U.S. Mobile Operator
Significant contract win with one of the U.S.'s largest mobile network operators covers provision of mobile marketing
29 July 2008
Simply Wireless Deploys Mobile Programs with 2ergo
Wireless retailer to use 2ergo's Via and Swift mobile technology products to power mobile marketing initiatives
15 July 2008
Proteus Now Re-Branded to 2ergo
U.S. re-brand strengthens 2ergo's position as a global organisation
10 June 2008
2ergo Launches Via, a Mobile Messaging Product
Self-service messaging product helps marketers quickly develop innovative mobile marketing campaigns
Find more news on the Web at www.2ergo.com.
31 July 2008
2ergo Secures Deal with Major U.S. Mobile Operator
Significant contract win with one of the U.S.'s largest mobile network operators covers provision of mobile marketing
https://web.archive.org/web/20150221055100/http://www.proteus.com/company/mission-statement
https://web.archive.org/web/20171115230727/http://www.proteus.com/evidence/
https://web.archive.org/web/20101124163357/http://www.accessdata.fda.gov/cdrh_docs/pdf9/K093976.pdf
…
This is not a "vaccine" but an offensive weapon system
Previous posts related to this subject:
https://outraged.substack.com/p/rfid-and-fda
https://outraged.substack.com/p/microchipped-and-marked-part-1
https://outraged.substack.com/p/microchipped-and-marked-part-2
https://outraged.substack.com/p/ingestible-sensors
https://outraged.substack.com/p/microchipped-and-marked-part-3
https://outraged.substack.com/p/bill-gates-is-a-pcr-test-fortune
https://outraged.substack.com/p/bill-gates-funds-creation-of-thin

















they still have nothing to track the pitchforks....
It is about them hoping to hack human consciousness…and perhaps master “time and space” in ways we do not currently understand. And all bets are off as to how far their covert explorations have taken them in their pursuit of essentially immortality. Their hubris knows no limits.